Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2011) Volume 2, Issue 3
Three simple, reliable methods were developed for the simultaneous determination of a mixture of Ambroxol HCl (A) and Guaifenesin (G) in presence of the oxidative degradate (AD) of A and guaicol (GD), the impurity of G . The first method is an isocratic HPLC method, where separation of the four components A, AD, G and GD was achieved on C18 column using water: methanol (80: 20, v/v, containing 1% triethylamine, pH 2.9) with a flow rate of 1.5 ml/min and UV detection at 220nm. A linear relationship in the range of 5-80 μg.ml -1 and 10-150 μg.ml -1 was obtained for A and G, respectively. The second method was a TLC-spectrodensitometric method, where the drugs together with AD and GD were applied on silica gel 60F 254 plates and a mobile phase consisted of chloroform: methanol: ethyl acetate: acetic acid (70: 8: 12:10, by volume) was used for separation. Densitometric evaluation of the separated zones was performed at 270 nm. The regression analysis for the calibration plots showed good correlation over the range 1-7 μg/ band and 2-10 μg/band for A and G, respectively. The third method was a multivariate spectrophotometric calibration method where principal component regression (PCR) and partial least squares (PLS) methods were used for the determination of the four components A, AD, G, and GD. The three methods were applied to pharmaceutical dosage forms containing either ambroxol alone (drops, capsules and tablets) or A together with G (syrup). Model update of multivariate calibration was used to determine A and G in syrup dosage form due to interfering additives. Results for the three methods were statistically compared with those obtained by applying reference reported methods for the drugs and showed that the proposed methods are accurate, precise, and can be easily applied.
Ambroxol (A) is a metabolite of bromhexine and is used similarly as a mucolytic, while Guaifenesin (G) increase the volume and reduce the viscosity of tenacious sputum and is used as an expectorant for productive cough [1]. Both drugs are present together in pharmaceutical dosage forms for the treatment of cough.
Several methods have been reported for the determination of ambroxol HCl, which include titrimetric methods [2], spectrophotometry [3,4], colorimetry [5], Flow injection analysis [6], HPLC [4,7,8], GC [4,9], CE [10], sequential injection chromatography [11], TLC [12,13] and voltammetry [14].
Analytical procedures for the determination of guaifenesin include titrimetry [2], spectrophotometry [15], colorimetry [16], Fluorimetry [17], HPLC [18-23], GC [24], CE [10,25], supercritical fluid chromatography [26] voltammetry [27] and chemometry [18,19,28,29].
Three HPLC for the determination of ambroxol and guaifenesin together with other drugs were reported [30-32]. This paper presents three methods for the simultaneous determination of ambroxol HCl and guaifenesin in presence of guaicol the impurity of guaifenesin [2] and the oxidative degradation product of ambroxol [12].
Apparatus
For HPLC: The HPLC system consisted of a two pumps LC-20 AT prominence Liquid chromatograph (Shimadzu, Japan) pump. Control unit DGU-20 A3 prominence Degasser (Shimadzu, Japan), SPD-20A prominence UV/Vis detector set at 220 nm, SIL-20A prominence Autosampler so that and a Waters Bondapack C18 column (4.6 x 250 mm) with a particle size of 10 µm.
For TLC: spectrodensitometry, CAMAG Linomat 5, autosampler (Switzerland), TLC aluminium plates, pre coated with silica gel 60 F254 (20 x 20cm), 0.2 mm thickness (Macherey-Nagel, Germany), CAMAG microsyringe, 100 µl (Switzerland), Glass chamber (Macherey- Nagel, Germany), UV Lamp-short wavelength 254 nm, CAMAG TLC Densitometric Scanner 3S/N 130319 with WINCATS software (CAMAG, Muttens, Switzerland).
For multivariate calibration: Dual-beam UV-Visible spectrophotometer, Jenway, 6800. The absorption spectra of the solutions were carried in 1cm quartz cells over the range of 200-400.
Software: All computations were performed in Matlab for WindowsTM version 6.5. The PLS procedure was taken PLS-Toolbox for the use with Matlab® 6.5.
Spectral characteristics: The absorption spectra of the mixtures of the four compounds (Amboxol HCL, Ambroxol HCl degradate, guaifenesin and guaicol) (Figure 1) were recorded over the range 200- 400 nm using methanol as blank.
Reagents and chemicals: Methanol-HPLC grade (E. Merck, Germany), Triethylamine and phosphoric acid 85% -Riedel-de-Häen, Sigma-Aldrich Labochemikalien GmbH (Germany), Bidistilled water, Methanol Analar and acetic acid-(SDFCL, s d fine-chem limited, Mumbai),Ethyl Acetate and chloroform-El Nasr Pharmaceutical Chemicals Co., Abu Zabaal, Cairo, Egypt.
Samples
Standards (pure samples): Ambroxol HCl- Assayed spectrophotometrically in 0.1M HCl and measured at 244 nm [4], it was found to contain 100.22 ± 0.754%, Guaifenesin- Assayed by HPLC [21] and was found to contain 100.28 ± 1.066%, Guaicol- Fluka, China contains not less than 98% guaicol by GC.
Both standards ambroxol HCl and guaifenesin were kindly supplied by Rameda Co. for Pharmaceutical Industries & Diagnostic Reagents, Cairo, Egypt.
Pharmaceutical preparations: Mucosin Syrup, manufactured by Rameda Co., Egypt. Each 5ml is labeled to contain 15 mg Ambroxol Hydrochloride and 100 mg Guaifenesin, Mucosin S.R. Capsules, manufactured by Rameda Co. for Pharmaceutical Industries & Diagnostic Reagents, Cairo, A.R.E. Each capsule contains 75 mg Ambroxol HCl, Ambroxol Tablets, manufactured by GlaxoWellcome Egypt S.A.E. Each tablet is labeled to contain 30mg Ambroxol HCl, Ambroxol Drops, manufactured by Glaxo Smithkline S.A.E., El Salam City, Cairo, A.R.E. Each100 ml contains 750 mg Ambroxol HCl.
Chromatographic conditions
For HPLC: The Waters column used was (4.6 x 250 mm) packed with Bondapack C18 with 10 µl particle size. The mobile phase consisted of methanol: water (20:80, containing 1 % triethylamine, pH adjusted to 2.9 ± 0.1 with phosphoric acid) was filtered through a 0.45 µm membrane filter and degassed by Ultrasonic vibrations prior to use, the pump was set at constant flow rate of 1.5 ml/min (isocratic elusion) and 20 µl of standards were injected (using an autosampler) on the column.
For TLC-UV densitometry: Samples were applied in the form of bands of 6 mm width with a 100 µl sample syringe on aluminium plates precoated with silica gel 60F254 (20 x 10 cm), using autosampler. A constant application rate of 0.1µl/s was used, and the space between bands was 12.1 mm. The slit dimension was 6.0 x 0.3 µm, and the scanning speed was 20 mm/s. The mobile phase consisted of chloroform-methanol-ethyl acetate-acetic acid (70:8:12:10, by volume), and 100 ml mobile phase was used / chromatographic run. Linear ascending development was carried out in a glass chamber saturated with the mobile phase. Development of the plates was left till the mobile phase migrates 9.5 cm. Following the development, the plates were air dried, spots were visualized under UV lamp at 254 nm and densitometric scanning was performed using CAMAG TLC Scanner in the reflectance-absorbance mode at 270 nm and operated by WINCATS software. The radiation source was deuterium lamp.
Stock solutions and calibration
Stock solutions for chromatographic methods: Stock standard solution of Ambroxol HCL (1mg.ml-1) (A) and Guaifenesin (1 mg.ml-1) (G) were prepared by weighing accurately 50 mg of pure A and G standards, in two separate 50 ml volumetric flasks, each drug was dissolved in about 30 ml methanol and the volume of the two flasks was completed to the mark with methanol.
Preparation of Ambroxol degradation, 0.5 g of ambroxol HCl was heated with 10 % H2O2 for 30 min, the solution is left to evaporate in air till dryness and the yellow powder is spotted on TLC plates to make sure that ambroxol HCl is completely oxidized. IR and Mass spectroscopy were also done for the degradation product (Figure 2 (included as supplementary data)). Standard Stock Solution of Ambroxol degradate (AD) was prepared by weighing 50 mg of AD in a 50 ml volumetric flask, the degradation was dissolved in 30 ml methanol and the volume was completed to the mark with the same solvent.
Calibration for HPLC: Construction of calibration curves for the determination of A and G: Aliquots equivalent to 50-800 µg and 100- 1500 µg, were transferred from the stock standard solutions of A and G, respectively, into two series of 10 ml volumetric flasks. Each volume was completed to the mark with methanol, and 20 µl was injected in triplicates onto the LC column. Relative peak area values were then plotted as a function of analyte concentration. The regression equation for each drug was computed from the graphs.
Calibration for TLC-spectrodensitometric method: Construction of calibration curves for the determination of A and G: Aliquots of standard stock solutions (1mg.ml-1) equivalent to (1-7 µg/band) for A and (2-10µg/band) were directly applied from the stock solutions of A and G on the silica gel plates, the plates were developed in the mobile phase (chloroform-methanol-ethyl acetate-acetic acid, 70:8:12:10, v/v/v/v) to 9.5 cm. The plates were then removed, visualized under UV lamp at 254 nm and scanned at 270 nm. The calibration curves representing the relationship between the relative peak area and the corresponding concentration were plotted and the regression equations were computed.
For multivariate calibration
Standard solutions: Stock standard solutions of Ambroxol HCl (A), Ambroxol HCl degradate (AD), Guaifenesin (G) and Guaicol (GD) were prepared by weighing accurately 0. 375 gm, 0.15gm, 0.50 gm and 0.50 gm of the four components, respectively, in four separate 100ml volumetric flask, the compounds were dissolved in 60 ml methanol and the volume was completed to the mark with the same solvent.
Working standard solutions were prepared by transferring accurately 2.5 ml of each of the stock standard solutions of A, AD and GD and 12.5ml of G, by pipette into four separate 250 ml volumetric flasks, the volume was completed to the mark with methanol and the flasks were shaken well.
Calibration: Multilevel multifactor design was used for the construction of calibration set [33]. A calibration set of 17 samples was prepared for the calibration. A five-level, four-factor calibration design was used in which 4, 4.5, 5, 5.5 and 6 ml aliquots of the four working standard solutions were combined in different mixtures and diluted to 25 ml with methanol in a 25 ml measuring flask. The absorbance of each mixture was scanned between 200-400 nm against a blank of methanol.
Application to pharmaceutical preparations
Accurate volume of mucosin syrup (10ml) was transferred and diluted in a 100 ml volumetric flask with methanol. Contents of twenty capsules and twenty tablets were accurately measured and powdered, separately. An accurate weight of each of the powdered sample of the tablets and capsules was transferred into two 25 ml volumetric flasks and the volume was completed to the mark with methanol. The two flasks were sonicated for 30 min., and then filtered through a filter paper previously wetted with methanol.
One ml of ambroxol drops was accurately measured and transferred into a 25 ml volumetric flask, the volume was completed to the mark with methanol, shaken well and filtered through a filter paper previously wetted with methanol.
For all dosage forms, suitable dilutions were made according to the linearity range, then the procedure detailed under linearity and calibration for each method was followed.
For HPLC Method
This paper describes the simultaneous determination of A and G in presence of guaicol, an impurity of guaifenesin and an oxidative degradation product of A. Three methods, namely, HPLC, TLCUV densitometry and multivariate calibration were used for the determination of the two drugs without any interference from their degradations or impurities, furthermore, the methods were applied to the pharmaceutical dosage forms.
For system optimization in case of HPLC, several columns and several systems were tried, however, depending on Literature review and Jain et al. [31] method; a new method was developed for the determination of both drugs with suitable retention times and without interference from their degradations or impurities using a mobile phase consisting of methanol: water (20:80, containing 1 % triethylamine, pH adjusted to 2.9 ± 0.1 with phosphoric acid). Increasing the ratio of methanol in the system, made the two peaks of G and GD overlap, while increasing the ratio of water, increased the retention time of A greatly above 25 minutes, the wavelength was also changed and 220 nm was chosen as it gave the highest response for both drugs. The retention times were 3.93, 8.44, 10.3 ± 0.1, 16.3 ± 0.1, for AD, GD, G and A, respectively (Figure 3).
Figure 3: a. Chromatogram of laboratory prepared mixture of Ambroxol HCl (50μg/ml) eluted at 16.345min, Guaifenesin (50μg/ml) at 10.4 min, Ambroxol HCl degradate (30μg/ml) at 3.93min and Guaicol (5μg/ml) at 8.4min. b. Chromatogram of Mucosin syrup with Ambroxol HCl (15μg/ml) and Guaifnesin (100μg/ml) together with two other peaks of the additives at 4.9 min and 12.7 min.
For TLC spectrodensitometric method: For TLC separation, several systems were tried. Encountering ammonia in the system, chloroform-methanol-ammonia (9.2:0.5:0.3, by volume), increased the Rf of ambroxol greatly and there was no complete separation between ambroxol and its degradation. The system chloroform-methanol-ethyl acetate-acetic acid (7;0.8:1.2:1, by volume) was used and it was found to be the optimum system for the separation of the two drugs in presence AD and GD, and this system was used in our determination of both drugs.
Scanning was tried at two wavelengths 270nm that is close to the maximum absorption of G and 254, which is close to the maximum absorption of A, the two drugs showed response at both wavelengths, however 270 nm was preferred. Saturation of the glass chamber with the mobile phase was also important; it was found that 45 minutes were enough for saturation. The plates were developed for a distance of 9.5 cm, which took approximately 30 minutes. Rf was found to be 0.14, 0.66, 0.79 and 0.88 for A, G, AD and GD, respectively, as shown in (Figure 4).
The calibration curves were constructed by plotting the relative peak areas (using 2 µg/band for A and G as external standard) versus the corresponding concentrations for each of the two drugs.
The proposed HPLC and TLC-spectrodensitometric methods were applied successfully to the analysis of the two drugs in different pharmaceutical dosage forms. Results were summarized in Table 4.
| HPLC | TLC-UV Densitometry | ||||||
| Parameter | A | G | Reference values | Parameter | A | G | |
| retention time tR | 16.3 ± 0.1 | 10.3 ± 0.1 | Rf | 0.14 | 0.66 | ||
| Resolution Rs | 9.86 | 2.63 | Rs≥2 | Resolution Rs | 7.7 (calculated between A and G) 2.27 (calculated between G and AD) |
||
| Tailing Factor | 1.11 | 1.2 | T≤2 | Capacity Factor (K') | 3.5 | 22.75 | |
| Asymmetry Factor | 1.33 | 0.98 | Selectivity α | 6.50 | |||
| Capacity Factor (K') | 6.13 | 3.52 | 1 |
Tailing Factor | 1.25 | 1 | |
| Selectivity α | 1.33 | 1.74 | α>1 | ||||
| Column Efficiency N | 2988.4 | 4261 | N>2000 | ||||
| HETP (Height Equivalent to theoretical plate) | 0.084 | 0.059 | |||||
Table 1: System suitability parameters for Ambroxol HCl and Guaifenesin by the proposed HPLC and TLC-spectrodensitometric method.
Multivariate calibration method: Two multivariate methods were applied for the determination of A, AD, G and GD, namely, PLS and PCR. These multivariate calibrations were useful as other spectrophotometric methods as derivative and derivative ratio failed to resolve the severe overlap in the absorption spectra of the four drugs, (Figure 5).
Mixtures of different concentrations (calibration set) of the four components (A, AD, G and GD) were used as calibration samples to construct the models. The spectra of these mixtures were collected and examined.
The selection of the optimum numbers of factors for PLS and PCR techniques was very important step before constructing the models because if the numbers of factors retained was more than required, more noise will be added to the data. On the other hand, if the number retained was too small, meaningful data that could be necessary for the calibration might be discarded. Different ways could be used for determining the optimum number of factors. In this study, the leave one out cross validation method was used and the RMSEC values of different developed models were compared. The model selected was that with the smallest number of factors such that RMSEC for that model was not significantly greater than RMSEC from the model with additional factor. As the difference between the minimum RMSEC and other RMSEC values become smaller, the significance of each additional factor becomes smaller. The maximum number of factors used to calculate the optimum RMSEC was selected to be 10. The PLS model required 8 factors, while the PCR required 9 factors.
To validate the prediction of the suggested models, they were used to predict the concentration of A, AD, G and GD in laboratory prepared mixtures (validation set), where satisfactory results were obtained (Table 2).
| Mix No | A µg.ml-1 | PLS | PCR | AD µg.ml-1 | PLS | PCR | G µg.ml-1 | PLS | PCR | GD µg.ml-1 | PLS | PCR |
| 1 | 7.50 | 99.29 | 99.40 | 2.40 | 98.85 | 98.95 | 40.00 | 102.51 | 102.15 | 12.00 | 98.57 | 98.58 |
| 2 | 9.00 | 99.44 | 99.46 | 3.00 | 95.90 | 95.76 | 45.00 | 99.03 | 99.05 | 9.00 | 100.74 | 101.07 |
| 3 | 6.75 | 99.10 | 98.87 | 2.70 | 99.62 | 99.69 | 55.00 | 101.50 | 102.04 | 12.00 | 96.99 | 96.49 |
| 4 | 6.75 | 101.91 | 101.83 | 3.30 | 101.32 | 101.38 | 60.00 | 100.93 | 101.08 | 11.00 | 100.81 | 100.72 |
| 5 | 7.50 | 100.18 | 100.25 | 3.60 | 100.22 | 100.29 | 60.00 | 98.24 | 98.03 | 8.00 | 106.21 | 106.56 |
| 6 | 6.00 | 101.01 | 100.78 | 3.00 | 101.47 | 101.30 | 55.00 | 100.77 | 101.22 | 11.00 | 99.40 | 99.59 |
| 7 | 8.25 | 100.76 | 100.79 | 3.30 | 99.81 | 100.12 | 45.00 | 99.60 | 99.50 | 8.00 | 100.85 | 100.09 |
| 8 | 8.25 | 101.7 | 101.74 | 2.70 | 100.72 | 100.97 | 40.00 | 99.39 | 99.23 | 9.00 | 101.12 | 100.77 |
| Mean | 100.42 | 100.39 | 99.74 | 99.81 | 100.25 | 100.29 | 100.59 | 100.48 | ||||
| R.S.D.% | 1.093 | 1.094 | 1.782 | 1.833 | 1.419 | 1.531 | 2.684 | 2.877 |
Table 2: Results of the analysis of the mixtures of the validation set of Ambroxol HCl, Ambroxol HCl degradate, Guaifenesin and Guaicol by the proposed PLS and PCR models.
The validation of the suggested models was done using several diagnostic tools. These tools were grouped into two categories, which were the model diagnostic tools used to determine the quality of the model and the sample diagnostic tools used to study the relationship between the samples and to identify unusual samples.
The predicted concentrations of the validation samples were plotted against the known concentration values (Table 3). This was used to determine whether the model accounted for the concentration variation in the validation set. Plots were expected to fall on a straight line with a slope of one and zero intercept. The four components lay on a straight line and the slope, intercept and correlation coefficient are shown in (Table 3). All plots had a slope nearly one and an intercept close to zero.
| Ambroxol HCl | Ambroxol HCl degradate | Guaifenesin | Guaicol | |||||
| Validation parameters | PLS | PCR | PLS | PCR | PLS | PCR | PLS | PCR |
| (a) Predicted vs. known concentration | ||||||||
| 1- Slope | 0.9795 | 0.9846 | 1.0282 | 1.0276 | 0.9935 | 1.0029 | 0.8840 | 0.9064 |
| 2- Intercept | 0.1793 | 0.1374 | -0.0917 | -0.0886 | 0.4375 | -0.0034 | 1.1865 | 0.9861 |
| 3- Correlation coefficient (r) | 0.9988 | 0.9989 | 0.9991 | 0.9988 | 0.9987 | 0.9980 | 0.9988 | 0.9994 |
| (b) Residual vs. actual concentration | 0.141 | 0.144 | 0.123 | 0.127 | 1.005 | 1.184 | 0.497 | 0.525 |
| ± error in prediction | ||||||||
| (c) RMSEP | 0.031 | 0.025 | 0.019 | 0.020 | 0.251 | 0.117 | 0.085 | 0.095 |
Table 3: Summary of results obtained by applying the diagnostic tools for model validation of the chemometric methods.
The concentrations of residuals were also plotted against the actual concentrations for the validation set samples for each component, (Table 3). This was used to determine whether the model accounted for the concentration variation in the validation set and it also provided information about how well the method would predict future samples. The residuals for all samples appeared to be randomly distributed around zero.
The RMSEP was a diagnostic tool for examining the errors in the predicted concentrations. It indicated both the precision and the accuracy of predictions. The RMSEP were calculated and the values were listed in (Table 3).
Both chemometric methods, PLS and PCR, were applied to the analysis of the pharmaceutical dosage forms. The two models were successfully applied to determine A in mucosin capsules, ambroxol tablets and amboxol drops , however, the two models gave recovery percentage higher than expected in case of mucosin syrup, due to interfering substances or additives .
Model updating
Multivariate calibration model can be updated [34] by including samples containing new sources of data variance, Xnew, to the existing calibration set, X, and the concentration of the new samples Ynew is added to the existing concentration matrix Y. This can be represented as follows:
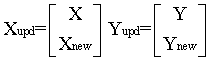
The updated model was capable of predicting the concentrations of A and G in mucosin syrup with good accuracy and precision without reconstruction of the model.
Model updating was applied to predict the concentrations of A and G in mucosin syrup which contain different spectral characteristics. The minimum number of samples necessary to update efficiently the model was also studied. The influence of the number of samples added to the calibration set on the RMSEP was studied for the developed multivariate model. One to ten samples containing A and G in the concentration range (6-9 µg.ml-1) and (40-60 µg.ml-1) for A and G, respectively were added to the initial calibration set. It was found that ten samples were necessary to perform an efficient update for PLS and PCR models for mucosin syrup. The predictive ability of the updated model was checked using external validation samples of the syrup dosage form (Table 4).
| HPLC | TLC-spectrodensitometry | PLS | PCR | ||||||
| Dosage form | Compound | Found ± RSD% | Recovery % of Standard addition | Found ± RSD% | Recovery % of Standard addition | Found ± RSD% | Recovery % of Standard addition | Found ± RSD% | Recovery % of Standard addition |
| Mucosin Syrup B. No. 07662 | Ambroxol HCl | 101.77 ± 1.095 | 100.87 ± 0.723 | 100.32 ± 0.550 | 100.43 ± 2.075 | 100.45 ± 1.821 | 100.33 ± 0.578 | 100.70 ± 1.684 | 98.78 ± 1.072 |
| Guaifenesin | 100.45 ± 1.067 | 100.50 ± 0.693 | 101.18 ± 0.653 | 102.06 ± 1.681 | 99.82 ± 1.880 | 100.47 ± 0.850 | 99.96 ± 1.986 | 101.70 ± 0.173 | |
| Mucosin Capsules B. No. 10301 | Ambroxol HCl | 99.01 ± 0.856 | 100.05 ± 1.298 | 99.45 ± 1.941 | 99.57 ± 2.006 | 99.47± 0.82 | 100.06 ± 0.418 | 100.13 ± 0.92 | 100.50 ± 0.500 |
| Ambroxol tablets B. No. 080078A | Ambroxol HCl | 99.71 ± 1.118 | 99.80 ± 1.997 | 99.34 ± 1.151 | 99.28 ± 1.731 | 100.13 ± 1.75 | 99.94 ± 0.587 | 100.80 ± 1.76 | 100.00 ± 1.00 |
| Ambroxol drops B. No. 091111A | Ambroxol HCl | 101.30 ± 0.440 | 100.37 ± 1.354 | 99.87 ± 0.987 | 99.28 ± 1.303 | 101.37 ± 1.52 | 99.89 ± 1.018 | 102.03 ± 1.44 | 100.11 ± 1.018 |
Table 4: Quantitative determination of Ambroxol HCl and Guaifenesin in pharmaceutical dosage forms by the proposed methods and application of standard addition technique
In order to assess the accuracy of the developed models, standard addition technique was carried out. Good mean recoveries indicate that the additives did not interfere with the determination of the two studied drugs (Table 4).
Method validation
Accuracy: The accuracy of the method was checked by applying the proposed methods for the determination of different pure samples of A and G. The concentrations were obtained from the corresponding regression equations.
The mean recovery percentages were 99.95 ± 0.821, 99.89 ± 1.029 for A and 100.09 ± 1.093, 100 ± 1.161 for G, for both methods HPLC and TLC spectrodensitometry (Table 5), respectively. The relatively low values of the relative standard deviation indicate the high precision of the two methods.
| Parameters | HPLC Method | TLC Spectrodensitometric Method | ||
| Ambroxol HCl | Guaifenesin | Ambroxol HCl | Guaifenesin | |
| Precision | ||||
| Repeatability | 1.388 | 1.984 | 0.851 | 1.274 |
| Intermediate precision | 1.577 | 2.082 | 1.807 | 1.699 |
| Calibration | ||||
| Range | 5-80 µg.ml-1 | 10-150 µg.ml-1 | 1-7 µg/band* | 2-10 µg/band* |
| Slope (a) | 0.0515 | 0.033 | 0.3220 | 0.2608 |
| Intercept (b) | -0.0414 | -0.012 | 0.3472 | 0.6443 |
| Correlation Coefficient (r) | 0.9999 | 0.9999 | 0.9999 | 0.9997 |
| Accuracy | 99.95 ± 0.821 | 100.07 ± 1.093 | 99.89 ±1.029 | 100.00 ± 1.161 |
| Specificity | 100.40 ± 0.964 | 100.00 ± 1.041 | 100.00 ± 1.179 | 99.90 ± 0.499 |
| LOD | 0.757 | 1.630 | 0.060 | 0.110 |
| LOQ | 2.295 | 4.941 | 0.182 | 0.334 |
Regression equations for Ambroxol HCl and Guaifenesin by TLC-spectrodensitometry followed second order equation:
For Ambroxol HCl, P= -0.0179C2+0.4652C+0.1324
For Guaifenesin, P= -0.0172C2+0.4677C+0.1386
Table 5: Validation results of the proposed HPLC and TLC-spectrodensitometric methods method.
Accuracy of the method was further assured by the use of the standard addition technique of known samples of pure A and G (three concentrations were used) to known concentrations of pharmaceutical preparations and the resulting mixtures were analyzed by the proposed methods. The results obtained were compared with the expected results. The good recoveries of the standard addition technique suggest good accuracy of the methods (Table 4).
Precision
Repeatability: The repeatability (intraday) was assessed by assaying three freshly prepared solutions of 20, 40 and 60 µg.ml-1 of A and 50, 80 and 100 µg.ml-1 of G in case of HPLC and of 2, 3 and 5 µg/band of A and 2,4 and 7 µg/band of G in case of TLC spectrodensitometry.
Inermediate precision (reproducibility): The intermediate precision (interday) was evaluated by assaying solutions of same concentrations as that of repeatability on three successive days. The results are summarized in Table 5.
Range: The calibration range was established through considerations of the practical range necessary according to the concentration range in pharmaceutical preparations to give accurate and precise results (Table 5).
Selectivity: Selectivity of the chromatographic methods was achieved by the analysis of different laboratory prepared mixtures of the two drugs within the linearity range containing different ratios of Ambroxol HCl degradation product and guaicol. Satisfactory results were obtained as shown in Table 5.
Stability: The stock solutions of the four compounds A, AD, G and GD showed no spectrophotometric changes for at least one week when stored in the refrigerator.
System suitability for chromatographic methods: Parameters including resolution (Rs), peak symmetry, capacity factor ((K/) and selectivity (α) were calculated and summarized in Table 1.
The method was further assessed by analyzing different samples and also by comparing the results with those obtained by applying reported methods [4,21]. Table 6 shows that the calculated t and F values are less than the corresponding tabulated ones, which proves that there is no significant difference between the suggested methods and the reported ones regarding accuracy and precision. However the proposed methods have the advantage where the two drugs A and G can be determined in presence of the degradate of A and the impurity of G without any interference. Also the three methods managed to determine A and G in presence of additives (benzoic acid) in syrup dosage form.
| Proposed methods for Ambroxol HCl | Reported methods for A | Proposed methods for Guaiphenesin | Reported methods for G | |||||||
| HPLC | TLC | PLS | PCR | Reporteda | HPLC | TLC | PLS | PCR | Reportedb | |
| Mean | 99.95 | 99.89 | 100.42 | 100.39 | 100.22 | 100.07 | 100.00 | 100.25 | 100.29 | 100.28 |
| RSD | 0.821 | 1.029 | 1.093 | 1.094 | 0.754 | 1.093 | 1.161 | 1.419 | 1.531 | 1.066 |
| Conc. Range | 5-80 µg.-1 | 1-7 µg.-1 | 6-9 µg.-1 | 6-9 µg.-1 | 10-150 µg/band | 2-10 µg/band | 40-60 µg.-1 | 40-60 µg.-1 | ||
| n | 5 | 7 | 8 | 8 | 4 | 6 | 9 | 8 | 8 | 7 |
| Variance | 0.674 | 1.059 | 1.195 | 1.197 | 0.569 | 1.195 | 1.348 | 2.014 | 2.344 | 1.136 |
| Student's t test | 0.508 (2.365)* | 0.556 (2.262)* | 0.325 (2.230)* | 0.325 (2.23)* | 0.350 (2.201)* | 0.496 (2.145)* | 0.046 (2.600)* | 0.014 (2.600)* | ||
| F-test | 1.185 (9.117)* | 1.861 (8.941)* | 2.100 (8.887)* | 2.104 (8.887)* | 1.052 (4.387)* | 1.187 (4.147)* | 1.773 (4.207)* | 2.063 (4.207)* | ||
n is the number of trials.
* These values represent the corresponding tabulated values of t and F at p=0.05.
a.Reported method was a spectrophotometric method for A by measuring the absorbance of A in 0.1M HCl at 244 nm.
b.HPLC for the determination of G on C18 column, mobile phase (KH2PO4-methanol, 55:45; v/v, pH3), Flow rate 1 ml/min UV detection at 220 nm.
Table 6: Statistical comparison of the results obtained by the proposed methods and the reference reported methods for pure samples of Ambroxol HCl and Guaifenesin.\
The proposed methods are methods were the two drugs can be determined in presence of each others as well as in presence of their degradation products or impurities. The developed methods were also successfully applied to syrup, drops, tablets and capsules dosage forms. The proposed methods are simple, specific and economic. TLC spectrodensitometry and multivariate calibration methods have the advantage of low operating costs, high sample output, and the need for minimal sample preparation. The major advantage of TLC-spectrodensitometric method is that several samples can be run simultaneously using a small quantity of mobile phase, thus reducing the analysis time and cost.