Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2022)Volume 13, Issue 2
Allyimidazolium based Bronsted acid ionic liquids with triflate ([ASBI][TfO]) and sulphate ([ASBI][HSO4]) anions, and it’s lewis acid derivatives ([ASCBI][TfO]) ([ASCBI][HSO4 ]) were synthesized to immobilize covalently on silica via anchoring thiol (-SH) functional group. The surface characterization of these silica immobilized ionic liquids was done by FTIR, TGA, elemental analysis, N2 surface area and acidity measurements. These high acidity SiO2 immobilized acid ionic liquids were used as bronsted and lewis acid catalysts in the esterification of glycerol with acetic acid, and found to have high selectivity for Diacetin (DAG) and Triacetins (TAG) compared with other established heterogeneous acid catalysts with excellent recyclability. To investigate the selectivity towards 1,3 diacetins and 1,2 diacetins, the acetylation reaction was studied at low conversions. The diacetin selectivity was also studied for different diols to compare with vicinal and terminal diols and found that diacetin selectivity is highly dependent on the chain length of the diols. The Bronsted acidic catalyst SiO2-[ASBI][HSO4], because of its high acidity was chosen to study the conversion and selectivity attributed to reaction parameters such as time, reaction temperature, catalyst loading and glycerol to acetic acid molar ratio.
Ionic liquids; SiO2 Immobilization; Glycerol; Diacetins
In the recent past, biodiesel production has gained considerable importance as potential replacement for petroleum fuels. The biodiesel synthesis involves transesterification of vegetable oils with lower boiling alcohols generating considerable amount of glycerol as byproduct [1]. The biodiesel production in 2011 was estimated at 8.6 and 4 million tons in Europe and USA, respectively [2,3]. This unwanted accumulation of glycerol has dropped its price, making it as a cost-effective building block for valuable chemicals and fuels additives [4].
The nontoxic, edible, biodegradable properties have made glycerol a versatile compound for wide range of applications, the presence of three hydroxyl groups in the molecule has focused the research on the catalytic conversion of bio-glycerol to high value chemicals such as 1,2-propanediol, 1,3-propanediol, acrolein, hydroxyacetone, glyceric acid and esters of glycerol[5]. Esterification of glycerol with acetic acid is one of the most potential approaches for the production of acetins, primarily used as precursors in the synthesis of polyesters [6,7]. In presence of acid catalyst at elevated temperature glycerol esterifies to Monoacetylglycerols or Monoacetins (MAG) which further esterifies to Diacetylglycerols or Diacetins (DAG) and Triacetylglycerol (TAG) or triacetins, with excess of acetic acid (Figure 1). Although monoacetins have valuable uses in pharmaceuticals, the importance of diacetins and triacetins as solvent is unique in industries and used as additives in transportation fuel.
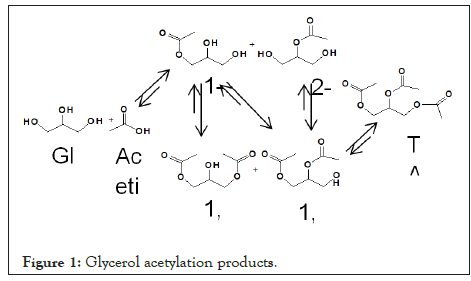
Figure 1: Glycerol acetylation products.
Several heterogeneous solid acid catalysts ranging from zeolites, supported acid catalysts, such as sulfated zirconia, and sulphated activated carbon, to mixed oxides like heteropolyacids, niobic acid, and hydroxylated magnesium fluorides, have been used for this reaction. Resin catalysts like amberlyst-15 and amberlyst-36 have been widely used for this reaction and compared with solid acid catalysts [8-15]. Funtionalization of acid groups (-SO3H sulphonic groups) on mesoporous silica (SBA-15, MCM-41), acidic resins have attracted much attention for this reaction [16,17]. Despite the relative efficiency of these catalysts in glycerol esterification, many of them have low densities of effective acid sites, tedious preparation protocols, and rapid loss of catalytic activity. The rate of esterification for monoglycerides being faster than diglycerides and triglycerides, most heterogeneous acid catalysts reported in literature show preferential selectivity for monoglycerides at intial reaction time. Although consecutive esterification of monglycerides leads to diacetins and triacetins, in some catalysts like mesoporous zeolite, crosslinked amberlyst polymers the rate of esterification towards diacetins and triacetins is much slower due to the hinder diffusion of bulky higher glycerides molecules in pores of mesoporous structures, leading to high selectivity towards monoglycerides. Even though esterification of glycerol has been widely studied, only few catalysts report high selectivity for diacetins and triacetins, moreover, reports on distribution of 1-Monoacetylglycerols (1-MAG), 2-Monoacetylglycerols (2-MAG), 1,2-Diacetylglycerols (1,2-DAG), 1,3-Diacetylglycerols (1,3-DAG) are very few.
Ionic Liquids (ILs), referred to a new class of green solvents due to negligible vapor pressure, has been fascinating as a catalyst in glycerol esterification [18]. Imidazolium (1-n-butyl-3- methylimidazolium tetrafluoroborate and hexafluorophosphate) based ILs are used as catalyst in esterification reaction primarily as bronstead acids and proven to be highly active, giving high selectivity towards desirable products. But, their applications as catalyst in industrial process are still hampered due to disadvantages like the product isolation and catalyst recovery with high purity. Also, ILs being expensive large amounts of ILs being used in homogeneous catalytic processes is not desirable.
To facilitate catalyst reuse, Ionic liquids can be immobilized on high surface area inorganic oxide surfaces like SiO2, which also minimize the consumption of ionic liquids in catalytic reactions, and find a promising use in esterification reactions, with higher performance. Immobilization of ILs has been tried on several high surface area porous materials like alumina, silica, activated carbon, zeolites and clays [19]. The most commonly used supports for immobilization are porous silica gels with a high surface area of 300-500 m2 g-1 due to presence of high density of hydroxyl groups.
The method of covalent anchoring of the anions by impregnation method and the successive immobilization of organic cation by ionic interaction has the disadvantage of leaching which could be avoided by the covalent bonding of the cation organic linker to the SiO2 surface and the substitution of anions..
Bronsted acidic ionic liquids containing sulphuric acid could be transformed to sulphonyl chloride to behave as Lewis acidic ionic liquids. Following this synthesis route, Yokoyama et al. reported the synthesis of Bronsted acidic ionic liquid, 3-allyl- 1-(4-sulphobutyl) imidazolium trifluoromethanesulphonate and its Lewis acid derivative, 3-allyl-1(4-sulphurylchloride butyl) imidazolium trifluoromethanesulphonate, and further immobilized 1-allylimidazolium based ionic liquids on modified silica gel via radical chain transfer reaction which proved to be recyclable heterogeneous acidic catalysts for esterification reaction. Although the allyl imidazolium immobilized SiO2 acid catalysts have proven to recyclable heterogeneous catalysts, their wide spread application in different esterification reactions and the relative study over acidity still remains to be explored.
In the present manuscript, we have grafted Bronsted and Lewis acid 3-allyl-1-(4-sulphobutyl) imidazolium based Ionic liquids with sulphate and triflate anion on high surface area silica. Herein, we also describe the application of these silica grafted Bronsted and Lewis acidic 3-allyl-1-(4-sulphobutyl) imidazolium ionic liquids (SiO2-ILs) as catalysts for the esterification of biodiesel glycerol with acetic acid to get glycerides. The distribution of products, selectivity of acetins based on Bronsted and Lewis acid SiO2-ILs also been studied and compared with amberlysts. The selectivity of diacetins have also been studied in esterification of different diols with SiO2-IL and amberlysts.
For synthesis immobilized acidic ionic liquid catalyst, Silica gel 60 (0.063~0.200 mm) was obtained from Merck Company. 3-mercaptopropyl-trimethoxysilane (3-MPS, 95%, Sigma-Aldrich), 1,4-butane sulphone, 99%, Sigma-Aldrich), 1-allylimidazole (99%, Alfa), azobisisobutyronitrile (AIBN, 98%, JUNSECI), thionyl chloride (99%, SAMCHUN Chemical) were used without further purification.
Glycerol (99%), 1,2 propanediol, 1,3 propanediol, 1,2 butanediol, 1,4 butanediol, 1,3 pentanediol, 1,5 pentanediol, 1,2 hexanediol and 1,6 hexanediol is a commercial product from Sigma-Aldrich, acetic acid from JUNSEI Company. Ionic liquid which is reaction solvent, 1-butyl-3-methylimidazolium chloride ([BMIM]Cl, 98%) and dimethylsulphoxide (DMSO, 99%) were obtained Sigma- Aldrich and JUNSEI Company. Amberlyst-15 and 36 acid resins were obtained from Sigma-Aldrich. ZSM-5 (Si/Al-50), Na form was obtained from Zeolyst and exchanged with acid to transform to H+ form.
Synthesis of immobilized acidic ionic liquid catalysts
Preparation of acidic ionic liquids: Acidic ionic liquids were prepared as per the reported procedures: 1-allylimidazole and 1,4-butane sulphone mixed in 250 mL flask at 273K for 24 hours. A solid formed was washed with diethyl ether, and then dried in a vacuum at ambient temperature. The resulting allyimidazolium was mixed with sulphuric acid and trifluoromethane sulphonicacid(1:1, mol/mol) at 313K. Acidic ionic liquids was obtained by washing with diethyl ether, and then dried in a vacuum for 2h. After drying, two bronstead acidic ionic liquids, 3-allyl-1- (4-sulphobutyl)imidazolium sulphate ([ASBI][HSO4]) and 3-allyl- 1-(4-sulphobutyl) imidazolium trifluoromethanesulphonate ([ASBI][TfO]) were obtained Figure 2. The resulting acidic ionic liquids when mixed with thionyl chloride (1:1, mol/ mol), -SO3H functional group changed to -SO2Cl functional group in imidazolium cation resulting into two lewis acidic ionic liquids, 3-allyl-1-(4-sulphurylchloride)butyl) imidazolium sulphate, ([ASCBI][HSO4 ]), 3-allyl-1-(4-sulphurylchloride)butyl) imidazolium trifluoromethanesulphonate ([ASCBI][TfO]) represented in Figure 2.
Figure 2: Synthesis of allylimidazole based acidic ionic liquid catalysts with different anions.
Immobilized acidic ionic liquids: Figure 3 shows the method of immobilization of acidic ionic liquids on silica. Firstly, thiol (-SH) functional group was immobilized on surface of silica gel. For that 20 g silica gel and 4.2 mL 3-MPS were dissolved in purified toluene for 2 hours. The precipitate was then washed with toluene for 12 hours in soxhlet extraction equipment.
Figure 3: Immobilization of the synthesized ionic liquids over silica.
Thiol grafted on silica surface (SiO2-MPS) was obtained by drying at 383K. For synthesizing immobilized acidic ionic lquid catalyst, 20 g SiO2-MPS, 0.02 mol AIBN and 0.02 mol acidic ionic liquid were dissolved in 150 mL purified acetonitrile for 30 hours. The precipitate was filtered, washed with acetonirile, acetone and diethyl ether to dry in vacuum at 383 K for 6 hours. The resulting immobilized acidic ionic liquid catalysts termed as SiO2-[ASBI][HSOHSO4], SiO2 -[ASBI][TfO] are bronstead acidic and the other SiO2-[ASCBI][HSO4], SiO2-[ASCBI][TfO] are lewis acidic.
The loading amount of acidic ionic liquids on silica gel was determined by element analysis. The nitrogen analysis of acidic ionic liquids (N, 4.1, 3.7, 4.0 and 3.8 mmol/g) grafted on the surface of silica gel was found to be similar.
Esterification reaction
The esterification of glycerol (0.01 mol) or diols with acetic acid (0.08 mol) was performed at atmospheric pressure in round bottom flask with a magnetic stirrer attached to a condenser and placed in an oil bath. 0.2 g of catalyst (5wt%) was introduced in to the reactor along with the glycerol. As soon as the reaction mixture reaction temperature, acetic acid was injected. The sampling was done at regular interval of time using syringe and were analyzed by gas chromatography (GC; Shimadzu 2010 chromatograph) fitted with HP-5 and DB-1 columns using Flame Ionization Detector (FID). The analysis of 1-MAG and 2-MAG was done with combination of both columns in GC. H1 NMR was also performed to analyze the distribution of the 1,2 DAG and 1,3 DAG.
Catalyst characterizations
FTIR, TGA and surface area of immobilized acidic ionic liquid catalysts: Thermogravimetric analysis (TGA-7, Perkin Elmer) was used to investigate the thermal and structural characteristics of the adsorbents. All the samples were carefully ground into fine power, and the temperature was increased from 323 to 873 K at a ramping rate of 10 K min-1 under inert N2 flow. A freshly activated catalyst powder sample was activated at 120oC for 12 h and the FTIR spectra of the samples were recorded on a Shimadzu (Model-820 PC) spectrophotometer.
The specific surface area of the catalysts was measured by N2 physisorption at liquid nitrogen temperature using a Quanta chrome Nova-1200 surface area analyzer and standard multi point BET analysis method. Samples were out gassed at 120°C in N2 flow for 12 hours before N2 physisorption measurements.
Acid strength of immobilized acidic ionic liquid catalysts: The acid strength of immobilized acidic ionic liquid catalysts were determined using Hammett Indicator. Firstly, 0.35 g of Hammett indicator was dissolved in 40 mL of dry benzene. Then 0.1 g of immobilized acidic ionic liquid catalysts were suspended in solution of Hammett indicator.
It has been compared with commercial acid catalyst (Amberlyst-15 and (Amberlyst-36). Acidity of immobilized acidic ionic liquid catalysts (SiO2-[ASBI][TfO], SiO2-[ASCBI][TfO], SiO2-[ASBI] [HSO4], SiO2-[ASCBI][HSO4]) has been observed in the range -4.4
Acid amount of immobilized acidic ionic liquid catalysts: The amount of acid on the immobilized acidic ionic liquid catalysts can be measured by amine titration. Samples suspended in benzene were titrated with n-butylamine in benzene using indiacator. The procedure is as follows: 0.1 g of dried catalyst was added to a 20 mL via 4.5 mL of dry benzene and 1.5 mL of indicator solution in benzene were added to catalyst suspension. Then n-butylamine in benzene was added quantitatively to sample and the calculated amount of acid in catalysts is given in Table 1. In results, commercial acid catalysts Amberlyst-15 and Amberlyst-36 showed acid amount of 4.6 and 5.06 mmol/g, respectively. For immobilized acidic ionic liquid catalysts the amount of acidity were observed in the range 1.89 to 2.12 mmol/g.
Characterization of immobilized acidic ionic liquid catalysts Figure 4 shows the FTIR spectrum of SiO2 immobilized ionic liquid catalysts, representing two characteristic peaks at the positions of 1640 and 1567 cm-1, which were attributed to the vibration peaks of allyl group and imidazole ring respectively. The Si–O absorption of silica can be observed as a strong peak at 1092 cm-1 whereas the weak absorption at 2937 cm-1 can be assigned to C–H bonds. Furthermore, the broad absorption in the region of 3440 cm-1 is due to the Si–OH groups on the surface of the silica. The peak at 796 cm-1 could be due to C-H bending. The FTIR result shows that the ionic liquids were successfully immobilized on SiO2 by covalent bonding of the cation organic linker.
Figure 4: FT-IR spectra of SiO2 immobilized catalyst; (a) SiO2- [ASBI][HSO4], (b) SiO2-[ASBI][HSO4], (c) SiO2-[ASBI][TfO], (d) SiO2-[ASCBI][TfO].
Figure 5 represents the TGA curve of the silica immobilized IL catalysts. All the SiO2 IL catalysts showed similar pattern of weight loss. The weight loss below 200oC for all the sample is attributed to water molecules adsorbed strongly on silica surface and the condensation of unreacted silanol groups.
Figure 5: Thermogravimetric curves of SiO2 immobilized catalyst; (a) SiO2-[ASCBI][TfO], (b) SiO2-[ASBI][TfO], (c) SiO2-[ASBI] [HSO4], (d) SiO2-[ASBI][HSO4].
Compared to triflate anion based catalyst, the weight loss in sulphate anion catalysts decreased significantly, which might be ascribed to the increased hydrophobicity of particle surface after immobilization of imidazolium sulphate ionic liquid. The weight markedly decreased above 300°C as the organic linkers on the silica gel decomposed completely around 700°C. The TGA profile shows that the SiO2 immobilied IL’s catalysts are stable untill 200oC.
Esterification results of glycerol and acetic acid over SiO2 immobilized acidic IL catalysts
Esterification results with heterogeneous catalysts: Table 1 contains the esterification results of glycerol with SiO2 immobilized acidic IL catalysts and well established heterogeneous amberlyst, and ZSM-5 acid catalysts. Table 1 shows turnover frequency (TOF) and product selectivity at 6 h of reaction time obtained with different catalysts used for esterification reaction. The surface area and acidity of the catalysts have also been incorporated. At first, blank test performed without catalyst with molar ratio of acetic acid to glycerol 8:1 and reaction temperature 110oC, glycerol conversion was 15% with 88% selectivity for MAG indicating the acidic protons from acetic acid is capable of catalyzing the reaction itself as suggested in literature. Under identical conditions, low surface area resins catalysts, like Amberlyst-15 (53 m2/g) and 36 (33 m2/g) shows 0.0015/hr TOF and the selectivity for MAG was 67% and 71%, respectively. Selectivity for DAG was low, 31% and 28%, for amberlyst-15 and 36 with 1% selectivity for TAG. With high surface area ZSM- 5 (354 m2/g), TOF was relatively high (0.0042/hr) and showed high selectivity for MAG, similar to amberlyst. Due to low acid sites density and configurational diffusion effects in the narrow pores, ZSM-5 shows lower performance than resins; in fact, their catalytic activity is only ascribed to mesopores in the borderline between adjacent zeolite particles.
| Catalyst | Surface area (m2/g) | Acid density | TOF. (%) | MAG (%) | DAG (%) | TAG (%) |
|---|---|---|---|---|---|---|
| (mmol/g) | ||||||
| Amberlyst-15 | 53 | 4.62 | 0 | 67 | 31 | 2 |
| Amberlyst-36 | 33 | 5.06 | 0 | 61 | 36 | 3 |
| H-ZSM-5(Si/Al-50) | 354 | 0.89 | 0 | 77 | 23 | 0 |
| Blank | - | - | - | 88 | 12 | 0 |
| SiO2 | 250 | 0.09 | 0.01 | 86 | 14 | 0 |
| SiO2-[ASBI][HSO4] | 281 | 2.12 | 0 | 24 | 60 | 16 |
| SiO2-[ASBI][TfO] | 290 | 1.94 | 0 | 27 | 57 | 16 |
| SiO2-[ASCBI][HSO4] | 260 | 2.01 | 0 | 18 | 64 | 18 |
| SiO2-[ASCBI][TfO] | 277 | 1.82 | 0 | 16 | 66 | 18 |
Note: Reaction conditions: molar ratio of acetic acid to glycerol=8:1, catalyst amount=5 wt% (w.r.t. glycerol), reaction time=6hr and reaction temperature=110oC.
Table 1: Glycerol acetylation with different silica immobilized ionic liquids as acid catalysts.
Bronsted and Lewis acid SiO2 immobilized catalysts had decent surface area mostly due to the silica itself, was also compared under identical conditions. Table 1 shows surface area of the catalysts increases slightly after IL immobilization on SiO2 but not much difference in surface area of catalysts immobilized with different IL’s. When utilized for esterification reaction, the Bronsted acidic SiO2-[ASBI][HSO4] catalyst showed TOF 0.0028/h of glycerol with only 22% selectivity for MAG, and significantly high selectivity of DAG (60%) and TAG (16%). The Lewis acidic SiO2-[ASCBI][HSO4] catalyst also shows 0.0029/hr TOF and even better selectivity for DAG (64%), and TAG (18%). Compared to the sulphate based IL catalysts the triflate based IL catalysts higher selectivity for DAG and TAG. SiO2-[ASBI] [TfO] and SiO2-[ASCBI][TfO] showed 0.0027/hr and 0.0029/hr conversion, with 57% and 66% selectivity for DAG, and 16% and 18% for TAG, respectively.
The activity of catalysts can be directly correlated to acid density in catalyst measured by titration, represented in Table 1. The titration shows that commercial resin catalysts, Amberlyst-15 and 36, has 4.62 mmol/g and 5.06 mmol/g acidity. The oxide based zeolite catalyst shows lower acidity, 0.89 mmol/g. The ionic liquids immobilized silica, SiO2-[ASBI][HSO4] and SiO2- [ASBI][TfO] shows high H+ acidity of 2.12 mmol/g to 2.01 mmol/g, not often reported in literature. Owing to high acidity and advantage of longer chain of immobilized ionic liquid group, silica immobilized IL catalysts show higher activity than resins. The lewis acid SiO2-[ASCBI][HSO4] and SiO2-[ASCBI][TfO] showed low acidity, 1.94 mmol/g and 1.82 mmol/g compared to the bronstead acid catalysts. Negligible acidity measured for SiO2 suggests that the acidity in SiO2 IL immobilized catalyst is generated mostly by the triflate and sulphate anions. The acidity in these catalysts is also due to the imidazole group and the grafted functional group in IL. Owing to it high TOF value SiO2 itself may seem to show good activity but the conversion of glycerol was only 17% after 6 hours and the reaction being self catalyzed upto 15%, the acid sites in SiO2 may not play a significant role in esterification. From Table 1, it’s interesting to observe that selectivity for DAG and TAG with SiO2 immobilized IL’s are much higher compared to commercial catalysts, particularly with lewis based SiO2-IL’s, SiO2-[ASCBI][HSO4] and SiO2-[ASCBI][TfO]. To investigate this interesting finding, reactions were performed with catalysts at lower conversions to check selectivity of acetins, and also analyze the distribution of isomeric monoacetins and diacetins, reported in Table 2.
| Catalyst | MAG Selec. (%)b | 1,2 DAG | DAG (1,3/1,2) b |
|---|---|---|---|
| Selection (%)b | |||
| Amberlyst-15 | 90 | 3 | 2.3 |
| Amberlyst-36 | 89 | 3 | 2.6 |
| SiO2-[ASBI][HSO4] | 78 | 5 | 3.4 |
| SiO2-[ASBI][TfO] | 75 | 6 | 3.1 |
| SiO2-[ASCBI][HSO4] | 70 | 7 | 3.2 |
| SiO2-[ASCBI][TfO] | 69 | 7 | 3.4 |
Note: Reaction conditions: molar ratio of acetic acid to glycerol=8:1, catalyst amount=5 wt% (w.r.t. glycerol) and reaction temperature=110oC. a Conversion at 6%, b Conversion at 25%
Table 2: Glycerol acetylation with different silica immobilized ionic liquids as acid catalysts.
Initially, reaction was monitored at low yield in order to notice the selectivity of 1 monoactein (1-MAG) and 2 monoacetin (2-MAG) but, avoid the formation of diacetins and triacetins. At 6% yield of monoacetins, with Amberlyst-15 and Amberlyst-36 the ratio of 1-MAG/2-MAG was 11.5 and 10.6, meaning better selectivity for 1-MAG with resins. The higher selectivity for 1-MAG suggests higher reactivity for hydroxyl group on primary than secondary position on glycerol. For all ionic liquid supported catalysts, the ratio of 1-MAG/2-MAG was less than resins, indicating increased selectivity for 2-MAG with SiO2 immobilized IL’s. The ratio of 1-MAG/2-MAG in lewis SiO2 -IL catalysts is noticeably higher than bronstead SiO2-IL catalysts, indicating different reactivity for the hydroxyl groups of glycerol, even with bronstead and lewis sites. From Table 1 results it seems hydroxyl group at secondary position is more accessible with SiO2-IL catalysts, particularly for bronstead SiO2-IL catalysts. Table 2 also shows the results of reaction monitored at around 25% conversion, performed inorder to study the consecutive esterification of monoacetins to diacetins but avoiding the further transformation to triacetins and specifically to analyze the distribution ratio of 1,3 diacetins (1,3-DAG) and 1,2 diacetins (1,2-DAG). At 25% conversion the selectivity for monoacetins is higher in all catalysts but with SiO2 immobilized IL’s there is significant decrease in selectivity for monoacetins compared to amberlysts. Theoretically, 1-MAG esterifies to 1,2-DAG and 1,3-DAG while 2-MAG can only transform to 1,2-DAG (Scheme 1). For amberlyst-36 catalysts the ratio of 1,3-DAG/1,2-DAG was 2.6 and the ratio of 1-MAG/2- MAG being 9.5 implies that considerable amount of 1-MAG transforms to 1,2-DAG. The diacetins selectivity ratio of all SiO2- IL was higher than amberlysts catalysts. Table 2 shows that the selectivity of diacetins with SiO2-[ASBI][HSO4] was 17% and the ratio of 1,3 DAG/1,2 DAG was 3.4, significantly higher than amberlyst, suggesting higher selectivity for 1,3 DAG. The ratio of 1-MAG/2-MAG dropping from 8.2 to 7.6, implies then transformation of 1-MAG to 1,3 DAG. For lewis acid SiO2- [ASCBI][HSO4] and SiO2-[ASCBI][TfO] the ratio was 3.2 and 3.4 meaning better selectivity (23% and 24%) for 1,3 DAG than 1,2 DAG. The higher ratio of diacetins with IL supported catalysts suggests that esterification of 1-MAG towards 1,3 DAG is much better with ionic liquids than amberlyst. The results indicates that due to high reactivity of hydroxyl group on primary carbons in glycerol a favorable selectivity to 1,3 DAG is obtained with silica imobilized ILs. This high reactivity could be due to the adsorption of carbonyl group of 1-MAG on the immidazolium ring giving high probability for the esterification reaction with the hydroxyl group on tertiary position instead of secondary position. This may leads to high selectivity for 1,3 DAG with SiO2 ionic liquid catalysts giving better selectivity for diacetins compared to the resin type of catalysts.
In a another study, esterification of linear diols with different chain length (C3 to C6 ) were also tested with amberlyst-15 and SiO2-[ASCBI][TfO] to find the reactivity of hydroxyl groups at terminal and vicinal position towards diacetins with catalyst. Figure 3 shows the ratio of di and mono acetins obtained at 25% conversion with catalytic conditions of 5 wt% catalyst at 110oC and at diol/ acetic acid ratio 1/8. In all reactions, the ratio of di/ mono acetins was less than 1 meaning selectivity of monoacetins is much higher than diacetins. From Figure 6, it can be seen that amberlysts-15 shows similar ratio of di/mono acetins for all vicinal diols (1,2). With amberlyst-15 as catalyst, the ratio of di/mono acetins for 1,3 propanediol is slightly higher than 1,2 propanediol and this difference in ratio of vicinal vs terminal gets bigger for Butanediol to Pentanediol and Hexanediol. This shows that as chain length increases the reactivity of terminal hydroxyl groups increases with amberlyst-15. While with SiO2-[ASCBI] [TfO] as catalysts, the ratio of di/mono acetins is higher than amberlyst-15 for 1,2 propanediol and much higher in case of 1,3 propanediol. This means better selectivity for diacetins with ionic liquid catalysts indicating better reactivity of hydroxyl groups on monacetins. Also, the difference in ratio of propanediol (1,2 vs 1,3) and butanediol (1,2 vs 1,4) is highest with SiO2-[ASCBI] [TfO]. These differences drops for pentanediol (1,2 vs 1,5) to hexanediol (1,2 vs 1,6) indicating that reactivity for terminal diols is much lower than vicinal diols. This shows that SO2 immobilized ionic liquids catalysts shows better reactivity for propanediol and butanediol but as chain length further increases the reactivity decreases. This specific activity could be due to the adsorption of carbonyl group of monoacetins on the immidazolium ring and owing to the size of monoacetins, gives high probability for the esterification reaction with the hydroxyl group on C2, C3 and C4 position but not so much for C5 and C6 position.
Figure 6: Comparison of SiO2-[ASCBI][TfO] with Amberlyst-15 in esterification of vicinal and terminal Diols. Reaction conditions: temp 110oC, diol/acetic acid=1:8 (molar ratio), 5 wt% catalyst loading relative to diols.
Effect of temperature: Figure 7 shows the effect of temperature on conversion of glycerol. The temperature effect was studied with 5 wt% SiO2-[ASBI][HSO4] at glycerol/ acetic acid 1/8 ratio and it was found that with an increase in temperature from 80oC to 110oC, the conversion increases from 75% to 92%. Figure 8 also reveals that as the temperature increases, the selectivity of MAG decreases rapidly from 72% to 21% along with a rise in selectivity of DAG and TAG. This can be explained due to the enhanced subsequent esterification of MAG with acetic acid to DAG and TAG, at temperatures close to reflux. At elevated temperatures, the product distribution of DAG remains almost constant due to fast reaction rate and attainment of equilibrium.
Figure 7: Glycerol esterification as a function of temperature over SiO2-[ASBI][HSO4 ]. Reaction conditions: glycerol/acetic acid=1:8 (molar ratio), 5 wt% catalyst loading relative to glycerol.
Figure 8: Glycerol esterification as a function of temperature over SiO2-[ASBI][HSO4 ]. Reaction conditions: glycerol/acetic acid=1:8 (molar ratio), 5 wt% catalyst loading relative to glycerol.
Effect of acetic acid to glycerol ratio: Table1 represents the effect of variation of the molar ratio of the acetic acid to glycerol at 110oC with 5% SiO2-[ASBI][HSO4] after 4 hours. The glycerol conversion and selectivity during esterification of glycerol also depended on the molar ratio of acetic acid to glycerol varied from 1/2 to 1/10. At 2/1 ratio of acetic acid to glycerol, which is lower than the stoichimetric ratio, the conversion of glycerol is 75% and the selectivity of MAG is 85% without any formation of TAG. The conversion and the selectivity of DAG increased with an increase in the amount of acetic acid. When the molar ratio of acetic acid to glycerol exceeded 4/1, selectivity of MAG decreases rapidly, due to the subsequent esterification reaction, raising the selectivity of DAG and the formation of TAG. From 1/8 ratio of acetic acid the selectivity of the higher glycerides remains nearly constant, this behavior could be due to excess of acetic acid resulting in the proportional decrease in the amount of catalyst and a decrease of acidity in the reaction system.
Effect of catalyst amount: Figure 9 shows the conversion and selectivity results for the variation of catalyst concentration during esterification of glycerol. At 110oC with acetic acid to glycerol ratio 1:8, the SiO2-[ASBI][HSO4] amount was varied from 1 to 7.5 wt% with respect to glycerol. Even at 1wt% the catalyst gives very high activity due to the presence of active acid sites. With further increase in the catalyst amount the active sites increases and the conversion also increases. The selectivity of MAG decreases as the catalyst amount increase from 1wt% to 5wt% but any further increase the selectivity of the glycerides remains almost consistent.
Figure 9: Glycerol esterification as a function of catalyst loading of SiO2-[ASBI][HSO4]. Reaction conditions: 110 oC, glycerol/acetic acid=1:8 (molar ratio), catalyst loading relative to glycerol.
The selectivity also varied with the change in catalyst concentration. The selectivity also depends on the conversion of glycerol. The selectivity towards diacetin is predominant at high glycerol conversion. The variations in reaction parameters suggest that the glycerol conversion and selectivity dependent on reaction temperature, time, glycerol to acetic acid molar ratio and catalyst amount. The selectivity is mainly varied with reaction time. Apart from other parameters selectivity also depend on the glycerol conversion. At lower glycerol conversion, monoacetin formation is favored. As the conversion increases, the selectivity is changed from mono to di and triacetin.
Recyclability test: Recyclability tests were performed using SiO2-[ASBI][HSO4] and the results are presented in Figure 10. For the recyclability experiments, reaction performed for 4 hours at 110oC using 5 wt% catalyst was filtered and washed with ethanol to separate the catalyst.
Figure 10: Recyclability test of SiO2-[ASBI][HSO4] in glycerol esterification reaction. Reaction conditions: 110oC, glycerol/acetic acid=1:8 (molar ratio), catalyst loading relative to glycerol.
After drying at 100°C overnight, the recovered catalyst was reused in fresh reactions with identical conditions for the subsequent cycles. The activity of the reused catalyst in the first run showed that the conversion of glycerol and selectivity of DAG was high but lower than the fresh catalyst itself. The subsequent runs show that the catalyst does not show any significant deactivation under these reaction conditions. Leaching test was also performed after every reaction; the filtrate after recovering the catalyst was also tested for elemental analysis of sulphur which shows negligible amount of Sulphur, which shows that SiO2-[ASBI][HSO4] catalyst does not leach in acidic conditions at 110oC and is regenerable.
In the conclusion, immidizole based Bonsted and Lewis acid ionic liquids had been successfully immobilized on silica, with good thermal stability, high surface area and high acid density. All the SiO2 supported ionic liquids shows similar activity and diacetins selectivity higher than commercial resins like Amberlyst-15 and 36 in the esterification reaction of glycerol and acetic acid. The catalytic activities show direct correlation with the acidity of different heterogeneous catalysts studied. The lewis acidic catalyst SiO2-[ASCBI][TfO], possessing 1.86 mmoles/g of acidity, exhibited highest selectivity for DAG (66%) and TAG. Interestingly, the high selectivity for the DAG with SiO2 ionic liquid catalysts is due to the preferential esterification on terminal hydroxyl groups on glycerol with lewis acid catalysts compared to amberlysts catalysts. The SiO2 ionic liquid catalysts shows high diacetins selectivity for terminal hydroxyl groups than vicinal hydroxyl groups in 1,3 propanediol and 1,4 butanediol and higher than pentanediol and hexanediol. The IL immobilized on silica gel could be separated from the reaction mixture easily and reused without significant dwindling of its initial activity.
We declare that we have no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Park YB, Kasinatham (2022) SiO2 Immobiliz ed Imidaz olium Ionic Liquid as Acid Catalysts for Diacetins and Triacetins Synthesis in G lycerol Esterification. J Thermodyn Catal. 13:293.
Received: 28-Feb-2022, Manuscript No. JTC-22-16240; Editor assigned: 04-Mar-2022, Pre QC No. JTC-22-16240; Reviewed: 18-Mar-2022, QC No. JTC-22-16240; Revised: 23-Mar-2022, Manuscript No. JTC-22-16240; Published: 31-Mar-2022 , DOI: 10.35248/2157-7544.22.13.293
Copyright: © 2022 Park YB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.