
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)
This study is a retrospective cohort of 122 adult patients with SARS-CoV-2 diagnosed and managed at two mediumsized, tertiary private hospitals. The analysis includes demographic and socio-economic information, symptoms, comorbidities, laboratory test results, therapeutic management, clinical outcomes and complications.
COVID-19; Cohort study; Latin America; Hospitalization; Mortality
As of May 29th, 2020, there were over 2.6 million Coronavirus disease 2019 (COVID-19) cases and over 151 thousand deaths in the Americas.
Surpassing the U.S. and Europe, most new cases and deaths are now being reported in Latin America (LA), the new epicenter of the pandemic [1].
The first confirmed case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Colombia on March 6, 2020. By March 25th the government instituted a national lockdown.
We report the characteristics of patients diagnosed and managed at Clinica del Country (CC) and Clinica La Colina (CLC), two medium-sized, tertiary private Hospitals, located in the northern region of Bogota, the fourth largest urban center in LA with a population of 7.86 million.
This study is a retrospective cohort of 122 adult patients (≥ 18 years) with positive polymerase chain reaction tests for SARS-CoV- 2, seen at CC & CLC from March 12th to May 25th, 2020. Testing, triaging and clinical management procedures were adopted from national guidelines [2].
Analyses include demographic and Socio-Economic (SES) information, symptoms, comorbidities, laboratory test results, therapeutic management, clinical outcomes and complications.
Data was stratified by Ambulatory Care (AC), General Ward hospitalization (GW) and Intensive Care Unit (ICU). Two of 27 ICU-managed patients were referred to other hospitals and their outcomes unknown.
We report numbers (percentages) for categorical variables and medians (interquartile ranges) for continuous variables.
In-hospital mortality and discharge dispositions are reported as of May 29th, with 5.7% of the cohort still hospitalized. The CC institutional review board approved the project with a waiver of informed consent.
Of 2010 tests performed, 122 (6%) were positive for SARSCoV- 2. Of these, 26 (21.3%) were managed in the GW and 27 (22%) in the ICU.
The cohort median age was 46 years (33.8-57.3), 70 (57.4%) were women and 43 (35.2%) health workers (Table 1).
| Characteristics | All patients | Management setting | |||
|---|---|---|---|---|---|
| Socio-demographics | Total | Intensive Care Unit n (%) | General Ward n (%) | Ambulatory Care n (%) | |
| 122 | 27 (22.1) | 26 (21.3) | 69 (56.6) | ||
| Age, median (IQR) [range] | 46 (33.8-57.3) [21-103] | 61 (48-69) [26-91] | 54 (36.62) [26-103] | 37 (31-47) [21-80] | |
| Sex | |||||
| Female | 70 (57.4) | 12 (44.4) | 11 (42.3) | 47 (68.1) | |
| Male | 52 (42.6) | 15 (55.6) | 15 (57.7) | 22 (31.9) | |
| Insurance Type | |||||
| Contributive | 29 (23.8) | 3 (11.1) | 5 (19.2) | 21 (30.4) | |
| Private | 93 (76.2) | 24 (88.9) | 21 (80.8) | 48 (69.6) | |
| Education level b | |||||
| Basic | 2 (1.6) | 2 (7.4) | - | - | |
| Intermediate | 23 (18.8) | - | 5 (19.2) | 18 (26.1) | |
| High | 59 (48.3) | 15 (55.6) | 10 (38.5) | 34 (49.3) | |
| Socio-economic levelc | |||||
| Low | 13 (10.6) | 2 (7.4) | 4 (15.4) | 7 (10.1) | |
| Middle | 62 (50.8) | 14 (51.9) | 13 (50.0) | 35 (50.7) | |
| High | 18 (14.7) | 5 (22.2) | 4 (15.4) | 9 (13.0) | |
| Occupation | |||||
| Health workersd | 43 (35.2) | 2 (7.4) | 6 (23.1) | 35 (50.7) | |
| Frontline workerse | 19 (15.6) | 4 (14.8) | 6 (23.1) | 9 (13.0) | |
| Non-essential workersf | 60 (49.2) | 21 (77.8) | 14 (53.8) | 25 (36.2) | |
| Transmission mode | |||||
| Imported | 20 (16.4) | 4 (14.8) | 3 (11.5) | 13 (18.8) | |
| Related to imported | 58 (47.6) | 10 (37.0) | 8 (30.8) | 40 (58.0) | |
| Unknown/Community | 44 (36.0) | 13 (48.1) | 15 (57.7) | 16 (23.2) | |
| Charlson comorbidity index score, mean (SD) | 1.2 (1.9) | 2.5 (2.1) | 1.92 (2.5) | 0.4 (1.0) | |
| Clinical outcomes | |||||
| Disease severity | |||||
| Uncomplicated disease | 73 (59.8) | - | 6 (23.1) | 67 (97.1) | |
| Mild pneumonia | 9 (7.38) | - | 7 (26.9) | 2 (2.9) | |
| Severe pneumonia | 22 (18.0) | 9 (33.3) | 13 (50.0) | - | |
| ARDS | 18 (14.7) | 18 (66.7) | - | - | |
| Septic shock | 16 (13.1) | 16 (59.3) | - | - | |
| Mechanical ventilation | 17 (13.9) | 17 (63.0) | - | - | |
| Median ventilator days (IQR) [range] | 4.0 (0-16) [0-66] | - | - | ||
| Vasopressor support | 17 (13.9) | 17 (63.0) | - | - | |
| Median vasopressor days (IQR) [range] | 2.0 (0-11) [0-26] | - | - | ||
| Median Length of stay (IQR) [Range] | |||||
| General ward | 7.0 (3-10) [0-25] | 6.0 (4-8) [2-12] | - | ||
| Intensive care unit | 6.0 (3-19) [1-66] | - | - | ||
| Readmitted | 17 (13.9) | 2 (7.4) | 6 (23.1) | 9 (13.0) | |
| Died | 3 (2.5) | 3 (11.1) | - | - | |
| Complications | |||||
| Ventilatory failure | 17 (13.9) | 17 (63.0) | - | - | |
| Cardiac complicationsg | 11 (9.0) | 9 (33.3) | 2 (7.7) | - | |
| Multiple organ failure | 8 (6.5) | 8 (29.6) | - | - | |
| Acute kidney injury | 9 (7.4) | 8 (29.6) | 1 (3.8) | - | |
| Dialysis | 6 (4.9) | 6 (22.2) | - | - | |
| Neurological complicationsh | 4 (3.3) | 4 (14.8) | - | - | |
| Acute liver injury | 2 (1.6) | 2 (7.4) | - | - | |
| Concomitant bacterial infection | 10 (8.2) | 9 (33.3) | 1 (3.8) | - | |
| Concomitant fungal infection | 1 (0.8) | 1 (3.7) | - | - | |
| Initial laboratory measures, median (IQR)i | |||||
| White blood cell count, mm3 | 6575 (5320-8330) | 6700 (5000-10600) | 7100 (5300-8050) | 6050 (5580-7750) | |
| Absolute neutrophil count, mm3 | 4550 (3180-6910) | 5260 (3500-9400) | 4950 (3880-6540) | 3590 (3000-5460) | |
| Absolute lymphocyte count, mm3 | 1240 (900-1760) | 1000 (770-1510) | 1030 (870-1480) | 1760 (1360-2390) | |
| Ferritin, ng/mL | 863 (280-1502) | 1250 (323-1751) | 321 (247-1299) | - | |
| D-dimer, ng/mL | 340 (193-660) | 508 (260-777) | 263 (154-585) | 184 (100-440) | |
| C-reactive protein, mg/dL | 5.1 (0.6-11.3) | 11.1 (6.3-19.8) | 5.3 (1.3-11.3) | 0.4 (0.2-0.7) | |
| Lactate dehydrogenase, U/L | 262 (210-351) | 357 (284-438) | 243 (216-299) | 207 (190-224) | |
| Alanine aminotransferase, U/L | 34 (21-52) | 44 (29-56) | 33 (21-52) | 30 (21-76) | |
| Aspartate aminotransferase, U/L | 35 (28-53) | 37 (19-55) | 33 (26-44) | 33 (27-63) | |
| Total bilirubin, mg/dL | 1 (0-1) | 1 (0-1) | 1 (0-1) | 1 (1-1) | |
| Influenza A/B n (%) | 1 (0.8) | - | 1 (3.8) | - | |
| Respiratory syncytial virus n (%) | 2 (1.6) | 2 (7.4) | - | - | |
| Treatment | |||||
| Oxygen | 47 (38.5) | 26 (96.3) | 21 (80.8) | - | |
| Nasal cannula | 43 (35.2) | 22 (81.5) | 21 (80.8) | - | |
| High flow nasal cannula | 4 (3.3) | 4 (14.8) | - | - | |
| Hydroxychloroquine | 42 (34.4) | 25 (92.6) | 17 (65.4) | - | |
| Lopinavir/ritonavir | 41 (33.6) | 25 (92.6) | 16 (61.5) | - | |
| Anticoagulation | 39 (32.0) | 21 (77.7) | 18 (69.2) | - | |
| Enoxaparin | 34 (27.9) | 17 (63.0) | 17 (65.4) | - | |
| Dalteparin | 1 (0.8) | 1 (3.70) | - | - | |
| Unfractionated heparin | 3 (2.5) | 2 (7.40) | 1 (3.8) | - | |
| Fondaparinux | 1 (0.8) | 1 (3.70) | - | - | |
| Macrolides | 8 (29.6) | 3 (11.5) | |||
| Azithromycin | 4 (3.3) | 3 (11.1) | 1 (3.8) | - | |
| Clarithromycin | 7 (5.7) | 5 (18.5) | 2 (7.7) | - | |
| Corticoids | 6 (4.9) | 6 (22.2) | - | - | |
| Tocilizumab | 1 (0.8) | 1 (3.7) | - | - | |
Note: a In case of readmissions data from the most severe encounter is reported. Two ICU-managed patients were referred to other hospitals and their clinical outcomes are unknown; b Education level categorized as low (primary/middle school), intermediate (high school or technical school degree) and high (bachelors or post-graduate degree). Missing data (n=38); c Socio-economic level categories based on government classification; missing data (n= 29); d Health workers includes physicians, registered nurses, nurse assistants, surgical assistants, dentists, psychologists, respiratory therapists, occupational therapists, pharmacists, social workers, hospital general services and administrative staff; e Frontline workers based on government classification as those with occupations deemed essential requiring them to continue to come to work (i.e. bus drivers, grocery clerks, law enforcement and others); f Non-essential workers based on government classification as those with occupations deemed feasible to work from home. (i.e. lawyers, administrative assistants, architects and others); g Cardiac complication includes arrhythmia, QT prolongation, myocarditis and acute heart failure; h Neurological complications includes seizures and multifactorial encephalopathy; i Not all laboratory tests reported were taken to all the patients, these were requested in accordance with clinical judgment and national guidelines.
Table 1: Characteristics of adults with SARS-CoV-2 managed in two hospitals, Bogota, Colombiaa.
Most patients had private health insurance 93(76.2%) and lived in northeast neighborhoods (https://bit.ly/36VE9Sm).
The majority of patients presented with cough, fever and shortness of breath and 29 (23.8%) had diarrhea/vomiting. Overall, the most prevalent comorbidities were hypertension and metabolic diseases. Most patients requiring hospitalization were in the 45-64 years group (22%) (Figure 1).
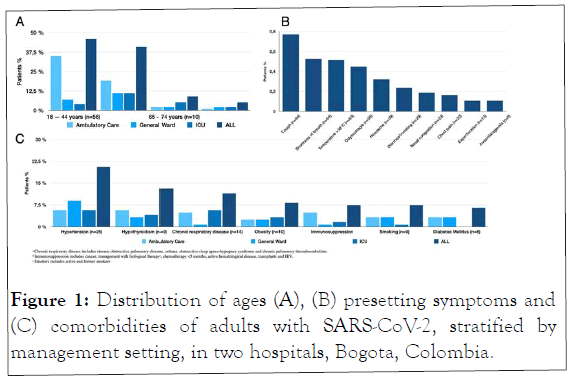
Figure 1: Distribution of ages (A), (B) presetting symptoms and (C) comorbidities of adults with SARS-CoV-2, stratified by management setting, in two hospitals, Bogota, Colombia.
Of the GW patients, 21 (80.8%) required oxygen support by nasal cannula. The primary reason for ICU admission was ventilator failure with a median length of stay of 6 (3-19) days and 17 (63%) requiring invasive mechanical ventilation.
To our knowledge, this is the first case series of patients with SARS-CoV-2 infection managed in outpatient and inpatient settings in Colombia and LA. With a median age of 46 years, our cohort is younger compared to series from China, Italy and the US [3-6]. This may be explained by the population age structure in Bogota and the early adoption of mitigation and suppression strategies, particularly protecting populations 70 and older. The median age and prevalence of comorbidities in our ICU-managed patients were similar to U.S series, with a modest mortality rate (11.1%).
The major limitation of this study is that our series mostly represents the earliest wave of SARS-CoV-2imported infections into the country, primarily among individuals of mid to high SES status. In our cohort, most patients 68 (64%) had a recent history of international travel or were related to imported cases. As the Bogota epidemic achieved sustained community transmission, subsequent infection waves have affected more frontline workers and lower SES status individuals. This is not surprising given the high levels of social inequality in large LA cities highlighting the importance of sustained local public health measures to reduce virus transmission and control the overall COVID-19 disease burden in the region.
Special thanks to Dr. Santiago Lopez, Medical Vice President CC and CLC and Dr. Alfonso Correa, Education and Research Subdirector CC and CLC for their support to conduct this study.
Author contributions
Drs. Moscoso, Sanchez and Rodriguez had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: Aya, Moscoso, Sanchez, Lobelo
Drafting of the manuscript: Moscoso, Sanchez, Gomez
Critical revision of the manuscript for important intellectual content: Rodriguez, Garzon, Lobelo
Statistical analysis: Sanchez and Moscoso
Obtained funding: N/A
Administrative, technical, or material support: Moscoso, Sanchez, Aya, Gomez
Supervision: Moscoso, Lobelo.
Conflict of interest disclosures: No disclosures to report
Citation: Moscoso DM, Sanchez S, Aya RNA, Gomez C, Rodriguez Y, Garzon J, et al. (2021) Socio-Demographic and Clinical Characteristics of Adults with Sars-Cov-2 Infection in Two Hospitals in Bogota, Colombia. J Clin Trials. S9:003.
Received: 19-Jan-2021 Accepted: 01-Feb-2021 Published: 08-Feb-2021 , DOI: 10.35248/2167-0870.21.s9.003
Copyright: © 2021 Moscoso DM, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.