
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2022)Volume 13, Issue 6
Introduction: Data from UNAIDS in 2021 pointed out that in Angola the number of individuals living with HIV is about 320,000. The system is the most exclusive group polymorphism is associated worldwide and has infected P.falciparum, P.vivax, Candida, H.pylori, HIV, V.B19, and Influenza Virus.
Objective: To evaluate the sociodemographic, clinical, and blood group profile among HIV patients in Luanda, the capital city of Angola.
Methodology: A descriptive, introspective, cross-sectional study with a quantitative approach was carried out with 130 patients from Luanda, in the second half of 2021.
Results: The 130 HIV patients included, the ORh+ group (46.9%) was the most predominant, followed by the BRh+ groups (25.4%) and ARh+ (23.1%). Female gender predominated (59,2%), with a basic level of education (58.5%), single (79.6%), employed (77.7%), living in Luanda Municipality (56.9%). Most patients who became infected having sex without a condom (91.5%), are in stage I of HIV infection (74,6%), and the number of TCD8 cells (20.8%) had an average (between 200 to 1000/mm), 20.8% of them were classified as group I (CD8 TBC greater than 500/mm), most individuals had a high viral load (between 100,000 and 1 million copies). According to the clinical history, the majority lived with the disease from 6 to 10 years (50%), 88.5% were on treatment with a retrovirus, and the majority used a combination of Tenofovir (TDF)+Lamivudine (3TC) or Emtricitabine (FTC)+Efavirenz (EFV) (TDF+3TC+EFV), most of them (54.7%) were undergoing treatment for less than 6 years, however, the statistical analysis showed no relationship between blood groups, and all analyzes (p>0.05).
Conclusion: HIV seems to be common in ORh+ individuals and seems to affect mainly women, young people, people with low education, who have a high viral load, infected for less than 6 years, where changes in the blood count occur moderately in individuals of groups O and A and the biochemical changes in individuals A, B and O.
HIV; ABO/Rh blood group; Sociodemographic and Clinical; Angola
Globally, the number of new HIV infections dropped by just 3.6% between 2020 and 2021, this was the smallest annual decline in new HIV infections since 2016. Eastern Europe and Central Asia, Middle East and North Africa and Latin America have seen the number of annual HIV infections increase over many years; and Asia and the Pacific which (the most populous region in the world) show that new HIV infections are also increasing where they were previously decreasing, however, East Africa and Austral, showed significant reductions in 2021 [1]. In West and Central Africa and the Caribbean, even reductions in HIV response have been threatened by an increasingly tight resource crisis [1,2]. Stagnant funding for the HIV response, along with global inequalities and the impact of the COVID-19 pandemic, is hampering progress in global programs to fight HIV, recent data indicate that current projections indicate that neither the targets of the UNAIDS 2025 95-95-95 nor the goal of ending the AIDS epidemic by 2030 will be met [2].
Data from UNAIDS in 2021 pointed out that in Angola the number of individuals living with HIV is about 320,000 [CI: 270,000-380,000], where adults aged 15 years and over are about 280,000 [CI: 240,000- 30,000] women in about 190,000 [CI: 160,000-220,000] and men in about 91,000 [CI: 77,000-110,000], children aged 0 to 14 years account for about 36,000 [CI: 28,000-46,000] [3].
The system is the most exclusive group.ax polymorphism is associated worldwide and has infected P.falciparum, P.vivax, Candida, H.pylori, HIV, V.B19, and Influenza Virus and also in cardiovascular diseases, hematological disorders, cognitive disorders, circulatory diseases, metabolic cancer and malaria [4,5]. A study of diseases of the group of patients 1 and 2 years earlier shows that they are susceptible to cognitive alterations such as hypertension, obesity, serious diabetes, previous diseases, and cardiovascular diseases and by E. cholera, plague, P susceptible to tuberculosis and mumps, while the similar group appeared to be more susceptible to smallpox and aeruginosa, and group B more susceptible to gonorrhea, tuberculosis, Streptococcus pneumoniae, E. coli, and salmonella [6].
Our research group, although with few resources, has carried out studies on blood groups in different diseases and has already found that in adult hypertensive individuals, the BRh+ group was more frequent (36.4%, n=36/99), in adult individuals with chronic kidney disease, the ORh+ group was more frequent (56.4%, n=79/140) in children with Nephrotic Syndrome (NS) and Sickle Cell Anemia (SC), the ORh+ group (42% and 60%, respectively), followed by the ABRh+ group (32% and 34%, respectively), in individuals submitted to hemoglobin electrophoresis the ORh+ and ABRh+ blood groups were the majority (40.6% and 28.9%, respectively) and in blood donors where the ORh+ group was the majority (61.8%) [7-11].
Despite several studies suggest that genetic factors like ABO and Rh blood groups would influence the occurrence of viral infectious diseases, no studies were found in Angola that assesses the sociodemographic, clinical, and blood group profile among HIV patients. From this perspective, the present study aimed to evaluate the sociodemographic, clinical, and blood group profile among HIV patients treated at the Josina Machel hospital, in the second semester of 2021, in Luanda, the capital of Angola.
Study design
An analytical, prospective study was carried out, with a quantitative approach, where the blood group (ABO system) and the Rhesus factor were determined in individuals with HIV treated at the hospital Josina Machel 2nd semester of 2021. Out of 300 patients, however, our sample was simply random, composed of 130 patients treated in the hospital unit and accepted to participate in the study, regardless of sex and age, which allowed us to work with a confidence index of 95% and a margin of error of 5%.
Ethics statement
To carry out the study, the project was submitted, analyzed, and approved by the Ethics Committee in Research on Human Beings of the Higher Institute of Health Sciences, University of Agostinho Neto (n.º 907/GD/ISCISA/UAN/2021) and by the Pedagogical and Scientific Department of Hospital Josina Machel-Maria Pia (nr. 204/DPC/HJM/2021). Before being included in the study, all patients had to sign an informed consent form, after being informed of the nature and objectives of the study.
Variables studied
Variables such as ABO/Rh blood group, age, sex, education, place of residence, type of infection, lifestyles, forms of contamination, associated diseases, complications results of the viral load, number of TCD8 and TCD4, viral load, number of TCD8 and TCD4, results of the erythrogram, leukogram and biochemistry tests.
Analysis of blood groups
A blood sample estimated at between 1 and 2 ml of blood was collected for each patient by the venipuncture technique and the samples were placed in test tubes containing Ethylene Diamine Tetraacetic Acid (EDTA). The samples were placed in three wells and the latter was associated with anti-A, Anti-B, and Anti-D reagents (Immucor, Portugal). Blood group determination was performed using the microplate technique according to the manufacturer's instructions, which is an agglutination test between the patient's serum and the anti-A, Anti-B, and Anti-D reagents from each well for phenotypic identification of the blood groups (ABO and Rh).
Statistical analysis
The data obtained in this study were analyzed using SPSS v20 (IBM SPSS Statistics, USA). Absolute and relative frequencies were determined in the descriptive analysis. Normally data distribution was expressed as mean and Standard Deviation (SD). Chi-square (X2) test was used to assess the relationship between categorical variables. All reported p-values are two-tailed and deemed significant when p<0.05.
Socio-demographic data:
The sociodemographic data found in HIV patients show that of the 130 HIV patients included in the study, most belonged to the ORh+ group, which represented 46.9% of the entire population studied, followed by the BRh+ groups (25.4% ) and ARh+ (23.1%). No Rh-individuals were found in the present study. The majority of the study population consisted of young people over 30 years of age, who together accounted for almost 83.1% of the accumulated percentage of the entire population, and only in the ABRh+ group was it found that more than 50% of the patients in this group were aged between 41 to 50 years. The female gender predominated among the individuals studied (59.2%) and, with the exception of the ABRh+ group, in all other groups the number of women exceeded 50% of the total of each group, the average age of men and women with HIV did not change much (42.5/43.3 years). The majority of patients with HIV had a basic level of education (58.5%), as the level of education increased, the incidence of HIV decreased, however, in all blood groups studied, the basic-level individuals represented percentages equal to or greater than 50%, an interesting fact was that the lowest average age among individuals with HIV was observed among those who had university education as their level of education (37.2 years). Single individuals represented 79.6% of the population studied and constituted more than 50% of the individual analysis of each blood group, in relation to marital status; married individuals with HIV had the highest mean age (51.9 years). Most HIV patients worked (77.7%), however, about 50% of ABRh+ individuals were unemployed, while 86.7% of ARh+ individuals had a job, as was to be expected in relation to the occupation of individuals with HIV, the lowest average age was observed in students (26.5 years). Regarding the place of residence, it was found that the majority was from the municipality of Luanda (56.9%), however, for the ABRh+ group and the ARh+ group, individuals from Luanda did not represent more than 50%. Statistical analysis showed no significant relationship between sociodemographic data and blood groups of HIV patients (p>0.05), regarding the organization of individuals with HIV by municipality of residence, the lowest average in all groups was greater than 40 years (Table 1).
| Blood groups | |||||||
| Age group | ABRh+ | ARh+ | BRh+ | ORh+ | N(%) | P-Value | Média (DP) |
| 6(4,6) | 30(23,1) | 33(25,4) | 61(46,9) | 130(100) | (X2) | 43,0 ± 10,9 | |
| <30 years | 1(16,6) | 3(10,0) | 7(21,2) | 11(18,0) | 22(16,9) | 0,770 | 26,4 ± 3,2 |
| 31 to 40 years | 1(16,6) | 7(22,3) | 10(30,3) | 13(21,3) | 31(23,8) | - | 36,9 ± 2,6 |
| 41 to 50 years | 3(50,2) | 14(46,7) | 10(30,3) | 19(31,1) | 46(35,4) | - | 45,4 ± 2,7 |
| >51 years | 1(16,6) | 6(20,0) | 6(18,2) | 18(29,5) | 31(23,8) | - | 57,3 ± 4,9 |
| Gender | |||||||
| Female | 2(40,0) | 22(73,3) | 20(60,6) | 33(54,1) | 77(59,2) | 0,185 | 43,3 ± 10,5 |
| Male | 4(60,0) | 8(26,7) | 13(39,4) | 28(45,9) | 53(40,8) | - | 42,5 ± 11,5 |
| Scholarly | |||||||
| Basic Education | 3(50,0) | 17(56,7) | 17(51,5) | 39(63,9) | 76(58,5) | 0,600 | 43,3 ± 10,4 |
| High school | 3(50,0) | 9(30,0) | 14(42,4) | 15(24,5) | 41(31,5) | - | 43,6 ± 11,9 |
| University education | 0(0,0) | 4(13,3) | 2(6,1) | 5(8,2) | 11(8,5) | - | 37,2 ± 9,5 |
| No schooling | 0(0,0) | 0(0,0) | 0(0,0) | 2(3,4) | 2(1,5) | - | 48,5 ± 12,0 |
| Marital status | |||||||
| Married | 1(10,0) | 3(10,0) | 5(15,2) | 11(18,0) | 20(15,4) | 0,793 | 51,9 ± 9,1 |
| Single | 5(50,0) | 26(86,6) | 27(81,8) | 45(73,8) | 103(79,2) | - | 41,0 ± 10,3 |
| Live marital | 0(0,0) | 1(3,4) | 1(3,0) | 5(8,2) | 7(11,4) | - | 46,4 ± 10,4 |
| Occupation | |||||||
| Unemployed | 3(50,0) | 4(13,3) | 3(9,1) | 8(13,1) | 18(13,8) | 0,060 | 42,1 ± 8,9 |
| Student | 0(0,0) | 0(0,0) | 5(15,2) | 6(9,8) | 11(8,5) | - | 26,5 ± 6,1 |
| Employed | 3(50,0) | 26(86,7) | 25(75,8) | 47(77,0) | 101(77,7) | - | 44,8 ± 10,0 |
| Municipality | |||||||
| Belas | 0(0,0) | 0(0,0) | 0(0,0) | 1(1,6) | 1(0,8) | 0,770 | 46,0 ± 0,0 |
| Cazenga | 0(0,0) | 1(3,4) | 4(12,2) | 1(1,6) | 6(4,6) | - | 42,5 ± 14,7 |
| K.Kiaxi | 0(0,0) | 6(20,0) | 2(6,1) | 4(6,5) | 12(9,2) | - | 40,7 ± 7,6 |
| Luanda | 2(30,0) | 14(46,7) | 22(73,3) | 36(59,0) | 74(56,9) | - | 42,9 ± 10,1 |
| Other | 1(20,0) | 0(0,0) | 0(0,0) | 5(8,2) | 6(4,6) | - | 46,7 ± 14,0 |
| Talatona | 0(0,0) | 1(3,4) | 4(13,3) | 2(3,5) | 7(5,4) | - | 41,6 ± 12,5 |
| Viana | 3(50,0) | 8(26,7) | 1(3,1) | 12(19,6) | 24(18,5) | - | 43,7 ± 13,3 |
Table 1: Sociodemographic data and blood groups of HIV patients.
When evaluating blood groups in relation to other diseases, complications in addition to HIV infection and consumption of alcohol and drugs, we found that most patients had no associated disease (70.8%), however, 29.2 % of these individuals had some other HIV-associated disease at the time of the study. The consumption of alcohol among all the individuals studied was higher than 20% and the use of drugs with the exception of individuals in the ABRh+ group was higher than 3%, as it was found that individuals who consumed other drugs were those with the lowest average age (39.5 years, SD=9.5). An interesting finding in this study was that among patients in the ORh+ group, individuals with associated diseases represented approximately 65.4%, which was not observed in other groups where individuals with associated diseases did not exceed 40% and the lowest mean age among the individuals studied, it was found among those who had sickle cell disease in addition to HIV (28, 2 years, SD=4.2). It was found that most patients included in the study had complications resulting from the disease (82.3%), however, digestive complications represented percentages above 10% among individuals in the ARh+ and ORh+ groups, and no individual in the ABRh+ had complications and the mean age between the different groups was between 40 and 47 years. There was no significant relationship between other diseases, complications, and alcohol and drug use with the blood group (p>0.05) (Table 2).
| Blood groups | |||||||
| Health status | ABRh+ | ARh+ | BRh+ | ORh+ | N(%) | P-Value | Age Mean ± SD |
| 6(4,6) | 30(23,1) | 33(25,4) | 61(46,9) | 130(100) | (X2) | 43(± 10,9) | |
| Alcohol and drugs | |||||||
| Alcohol | 2(33,3) | 6(20,0) | 8(24,2) | 18(29,5) | 34(26,2) | 0,969 | 41,5 ± 10,5 |
| Drugs | 0(0,0) | 1(3,3) | 1(3,0) | 2(3,3) | 4(0,8) | - | 39,5 ± 9,6 |
| None | 4(66,7) | 23(76,7) | 24(72,7) | 41(67,2) | 92(70,8) | - | 43,6 ± 11,0 |
| Associated diseases | |||||||
| Sickle Cell Anemia | 0(0,0) | 2(6,6) | 0(0,0) | 0(0,0) | 2(1,5) | 0,335 | 28,2 ± 4,2 |
| COVID-19 | 0(0,0) | 2(6,6) | 0(0,0) | 1(1,6) | 3(2,3) | - | 33,3 ± 14,4 |
| Diabetes mellitus | 0(0,0) | 0(0,0) | 0(0,0) | 2(3,2) | 2(1,5) | - | 55,5 ± 2,1 |
| Hepatitis B | 0(0,0) | 2(6,6) | 0(0,0) | 3(4,9) | 5(3,8) | - | 36,4 ± 4,7 |
| Herpes Zoster | 0(0,0) | 0(0,0) | 3(9,1) | 4(6,5) | 7(5,4) | - | 42,4 ± 14,1 |
| Hypertension | 1(20,0) | 1(3,3) | 1(3,0) | 0(0,0) | 3(2,3) | - | 52,3 ± 4,0 |
| Malaria | 0(0,0) | 1(3,3) | 3(9,1) | 3(4,9) | 7(5,4) | - | 41,0 ± 9,1 |
| Pneumonia | 0(0,0) | 1(3,3) | 1(3,0) | 0(0,0) | 2(1,5) | - | 54,5 ± 0,7 |
| Rheumatism | 0(0,0) | 0(0,0) | 1(3,0) | 0(0,0) | 1(0,8) | - | 36,0 ± 0,0 |
| Tuberculosis | 0(0,0) | 2(6,6) | 1(3,0) | 3(4,9) | 6(4,6) | - | 45,0 ± 9,7 |
| None | 5(80,0) | 19(63,3) | 23(69,7) | 45(34,6) | 92(70,8) | - | 43,3 ± 10,8 |
| Complications | |||||||
| Liverworts | 0(0,0) | 1(3,3) | 0(0,0) | 2(3,2) | 3(2,3) | 0,867 | 40,7 ± 14,2 |
| Nervous system | 0(0,0) | 0(0,0) | 1(3,0) | 0(0,0) | 1(0,8) | - | 47,0 ± 0,0 |
| Respiratory | 0(0,0) | 2(6,7) | 2(6,1) | 1(1,6) | 5(3,8) | - | 44,2 ± 10,7 |
| Digestive | 0(0,0) | 2(6,7) | 4(12,2) | 8(13,1) | 14(10,8) | - | 42,2 ± 11,4 |
| None | 6(100,0) | 25(83,3) | 26(60,1) | 50(81,9) | 107(82,3) | - | 43,0± 10,9 |
Table 2: Blood groups and history of diseases, complications and use of alcohol and drugs in patients with HIV.
When evaluating the blood groups and clinical history of individuals with HIV, we found that most patients became infected through having sex without a condom (91.5%), however, individuals in the AR+ group (6.7 %) and ORh+ (6.6%), did not know, reported that the form of HIV infection was not through unprotected sexual intercourse, and the lowest mean ages were observed in individuals who did not know how they became infected or who became infected in a different way. other than sexual intercourse (average age below 35 years). We noticed that most of the individuals studied were in stage I of HIV infection (746%), however, with the exception of the ABRh+ group, in all groups the percentage of individuals in stage II was greater than 16% and individuals in stage III were found only in patients in the BRh+ and ORh+ groups, and the lowest mean age was found exactly in individuals in stage III (365 years, SD=8.9). In the categorization of individuals with HIV in relation to the number of TCD8 cells, we noticed that more than 66.6% of the clinical files of the individuals studied did not contain information on the number of TCD8, but about 20.8% had TCD8 numbers considered average (between 200 to 1000/mm) and the lowest mean age was observed among individuals who had low TCD8 numbers (less than 200/mm), where the mean age was 38.5 years, SD=17.6. When classifying patients with HIV in relation to the number of TCD4 cells, we noticed that 75.4% of them did not contain this information in their clinical processes, although 20.8% of them were classified as group I (CD8 TBC greater than 500/mm), where, with the exception of the ARh+ group (13.3%), all other groups presented a percentage higher than 20% and the average age between the different categories by number of TCD8 was between 42 and 45 years. In classifying patients by viral load, we found that most individuals had a high viral load (between 100,000 and 1 million copies), which represented 66.9% of the population studied, and this percentage did not vary much between different blood groups, however. , the lowest mean age (34.7 years, SD=8.7) was observed among individuals with an undetectable viral load (<50 copies). There was no significant relationship between HIV clinical history and blood group (p>0.05) (Table 3).
| Blood groups | |||||||
| Clinical history | ABRh+ | ARh+ | BRh+ | ORh+ | N(%) | P-Value | Age Mean ± SD |
| 6(4,6) | 30(23,1) | 33(25,4) | 61(46,9) | 130(100) | (X2) | 43(± 10,9) | |
| Form of infection | |||||||
| Sexual | 6(100,0) | 26(86,7) | 32(97,0) | 55(90,2) | 119(91,5) | 0,707 | 43,7 ± 10,4 |
| Others | 0(0,0) | 2(6,7) | 0(0,0) | 4(6,6) | 6(4,6) | - | 34,6 ± 12,3 |
| Unknown | 0(0,0) | 2(6,7) | 1(3,0) | 2(3,3) | 5(3,8) | - | 34,4± 13,7 |
| WHO stadium | |||||||
| I (Asymptomatic) | 5(83,3) | 24(80,0) | 23(69,7) | 45(73,8) | 97(74,6) | 0,772 | 43,9 ± 11,1 |
| II (Weight loss<10%) | 1(16,7) | 6(20,0) | 7(21,2) | 12(19,7) | 26(20,0) | - | 40,9 ± 9,6 |
| III (Weight loss >10%) | 0(0,0) | 0(0,0) | 3(9,1) | 4(6,6) | 7(5,4) | - | 36,5 ± 8,0 |
| Number by TCD8 numbers | |||||||
| Low(<200/mm) | 0(0,0) | 0(0,0) | 0(0,0) | 2(3,3) | 2(1,5) | 0,773 | 38,5 ± 17,6 |
| Medium(200-1000/mm) | 1(016,7) | 4(13,3) | 8(24,2) | 16(26,2) | 29(22,3) | - | 41,1 ± 13,0 |
| High(˃1000/mm) | 1(16,7) | 4(13,3) | 2(6,1) | 5(8,2) | 12(9,2) | - | 49,5 ± 6,6 |
| Unknown | 4(66,7) | 22(73,3) | 17(69,7) | 36(62,3) | 71(66,9) | - | 41,7 ± 10,2 |
| Classification by TCD4 numbers | |||||||
| Group I(≥ 500/mm) | 2(33,3) | 4(13,3) | 7(21,2) | 14(23,0) | 27(20,8) | 0,659 | 45,2 ± 11,8 |
| Group II(200 to 499/mm) | 0(0,0) | 2(6,7) | 1(3,0) | 2(3,3) | 5(3,8) | - | 45,2 ± 11,8 |
| Unknown | 4(66,7) | 24(80,0) | 25(75,8) | 45(73,8) | 98(75,4) | -- | 42,1 ± 10,6 |
| Classification by viral load | |||||||
| High(≥ 10.000) | 5(83,3) | 18(60,0) | 22(66,7) | 42(68,7) | 87(66,9) | 0,811 | 45,1 ± 10,4 |
| Low(51-10.000) | 0(0,0) | 4(13,3) | 6(18,2) | 10(16,4) | 20(15,4) | - | 38,3 ± 11,3 |
| Undetectable(≤ 50) | 0(0,0) | 4(13,3) | 3(9,1) | 6(9,8) | 13(10,0) | - | 34,7 ± 8,7 |
| Unknown | 1(16,7) | 4(13,3) | 2(6,3) | 3(4,9) | 10(7,7) | - | 43,7 ± 8,9 |
Table 3: Blood groups and clinical history of HIV patients.
In the evaluation of the pharmacological history of patients with HIV, according to blood groups, we found that the majority of patients had already lived with the disease for 6 to 10 years (50%), however, the majority (more than 95%) of ARh+ and BRh+ patients lived with the disease between 1 and 10 years, which did not happen with ABR+ and ORh+ patients who lived with the disease for a longer time, and the lowest mean age was observed specifically in individuals who lived with the disease and less of 6 years (36.0 years, SD=9.4). We found that 88.5% of the individuals were on treatment with a retrovirus, however, 9.2% had interrupted the treatment, while 2.3% were not on antiretroviral treatment, among the blood groups, the ABRh+ patients were the ones that most interrupted the treatment (40%), plus the mean age difference between the groups of patients in relation to treatment was very small (from 40 to 43 years). Most patients were on a combination treatment of Tenofovir (TDF)+Lamivudine (3TC) or Emtricitabine (FTC)+Efavirenz (EFV) (TDF+3TC+EFV), and with the exception of individuals in the ARh+ group, more than 50% of patients from other groups used the aforementioned combination, while 2% had not yet received any treatment and were all of the ARh+ blood group, it was noticed that the lowest mean age was among those individuals who did not receive any treatment (35.5 years, SD=14.8). When evaluating the time in which the individuals included in the study were undergoing treatment, we found that most of them (54.7%) were undergoing treatment for less than 6 years, however, there were patients who were not yet undergoing treatment and it was in these two groups where we identified the lowest average age (less than 40 years). It was found that only 10.8% of patients had suspected failure in retroviral treatment, in individuals in the BRh+ and ORh+ groups, the percentage of individuals suspected of treatment was greater than 11 %, and the mean ages in this condition ranged from 41 to 43 years. However, the statistical analysis showed no relationship between blood groups and all analyzes of the drug history of individuals with HIV (p>0.05) (Table 4).
| Blood groups | |||||||
| Medication history | ABRh+ | ARh+ | BRh+ | ORh+ | N(%) | P-Value | Age Mean ± SD |
| 6(4,6) | 30(23,1) | 33(25,4) | 61(46,9) | 130(100) | (X2) | 43(± 10,9) | |
| Illness time | |||||||
| ≤ 5 years olds | 0(0,0) | 7(23,3) | 8(24,2) | 17(27,9) | 32(24,6) | 0,262 | 36,0±9,4 |
| 6-10 years olds | 3(50,0) | 13(43,3) | 15(45,5) | 34(55,7) | 65(50,0) | - | 43,8 ± 10,3 |
| 11-15 years olds | 2(0,0) | 8(6,7) | 9(0,0) | 10(0,0) | 29(22,3) | -- | 49,3 ± 9,7 |
| ≥16 years olds | 1(16,7) | 2(6,7) | 1(3,0) | 0(0,0) | 4(3,1) | - | 36,7 ± 7,1 |
| Administration of antiretrovirals | |||||||
| Discontinued treatment | 2(40,0) | 3(10,0) | 2(6,1) | 5(8,2) | 12(9,2) | 0,220 | 43,9 ± 9,1 |
| Does not do the treatment | 0(0,0) | 2(6,7) | 0(0,0) | 1(1,7) | 3(2,3) | - | 40,7 ± 13,8 |
| Doing the treatment | 4(60,0) | 25(83,3) | 31(93,9) | 55(90,1) | 115(88,5) | - | 42,9 ± 11,1 |
| Anti-retroviral treatment scheme | |||||||
| AZT+3TC+LPV/r | 1(20,0) | 5(16,7) | 5(15,1) | 5(8,2) | 16(12,3) | 0,477 | 44,3 ± 10,8 |
| AZT+3TC+NVP | 0(0,0) | 2(6,7) | 5(15,1) | 4(6,5) | 11(8,5) | - | 46,6 ± 9,6 |
| TDF+3TC+DTG | 4(60,0) | 14(46,6) | 17(51,5) | 36(59,0) | 71(54,6) | - | 42,3 ± 10,2 |
| TDF+3TC+EFV | 1(20,0) | 3(10,0) | 3(9,0) | 9(14,7) | 16(12,3) | - | 43,5 ± 12,4 |
| TDF+3TC+LPV/r | 0(0,0) | 0(0,0) | 1(3,0) | 4(6,5) | 5(3,8) | - | 47,6 ± 13,2 |
| TDF+3TC+NVP | 0(0,0) | 4(13,2) | 2(6,1) | 3(4,9) | 9(6,9) | - | 39,2 ± 13,8 |
| None | 0(0,0) | 2(6,7) | 0(0,0) | 0(0,0) | 2(1,5) | - | 35,5 ± 14,8 |
| Treatment time | |||||||
| ≤ 5 years olds | 2(33,3) | 15(50,0) | 17(51,5) | 37(60,7) | 71(54,6) | 0,602 | 39,3 ± 9,9 |
| 6-10 years olds | 1(16,7) | 5(16,7) | 7(21,2) | 14(23,0) | 27(20,8) | - | 47,0 ± 10,0 |
| 11-15 years olds | 3(50,0) | 9(30,0) | 9(27,3) | 9(14,8) | 30(23,1) | - | 48,0 ± 10,4 |
| None | 0(0,0) | 1(3,3) | 0(0,0) | 1(1,6) | 2(1,5) | - | 38,0 ± 18,3 |
| Suspected therapeutic failure | |||||||
| No | 6(100,0) | 28(93,3) | 28(84,8) | 54(88,5) | 116(89,2) | 0,582 | 43,1 ± 10,9 |
| Yes | 0(0,0) | 2(6,7) | 5(15,2) | 7(11,4) | 14(10,8) | - | 41,5 ± 10,8 |
Note: AZT+3TC+LPV/r- Is the combination of ZDV/3TC (Combivir®) and Lopinavir/ritonavir (LPV/r) (Kaletra® or Aluvia®). AZT+3TC+NVP- Is the combination of AZT+3TC (Lamivudine)+NVP (Nevirapine). TDF+3TC+DTG- Is the combination of Tenofovir (TDF)/ Lamivudina (3TC)+Dolutegravir (DTG). TDF+3TC+EFV- Is the combination of tenofovir (TDF)+lamivudine (3TC) or emtricitabine (FTC)+efavirenz (EFV). TDF+3TC+LPV/r- Is the combination of tenofovir (TDF)+lamivudine (3TC)+lopinavir/ritonavir (LPV/r). TDF+3TC+NVP- is the combination of tenofovir (TDF)+lamivudine (3TC) or emtricitabine (FTC)+NVP (Nevirapine).
In the leukogram evaluation, individuals in the group of all blood groups had a mean percentage of neutrophils below the reference values (below 50%) and individuals in the ABR+ blood group showed a slight decrease in the mean number of neutrophils followed (1.4/µL ) slightly below the reference values. Individuals in the ABR+ blood group showed a slight decrease in the mean percentage of lymphocytes (more than 41%). Individuals in the ORh- blood group had increased means of monocyte percentage (12.59±27.99) and eosinophil percentage (5.5±6.92). In the erythrogram evaluation, we noticed that individuals in the ARh+ and ORh+ groups showed a slight reduction in the average number of hematocrits (less than 37%), and all other parameters were in normal averages in all blood groups. The average number of platelets in all blood groups was within the reference values (Table 5).
| Grupos Sanguíneo | ||||
| Blood count | ABRh+ Mean ± SD | ARh+ Mean ± SD | BRh+ Mean ± SD | ORh+ Mean ± SD |
| n=6(4,6%) | n=30(23,1%) | n=33(25,4%) | n=61(46,9%) | |
| Leucogram | ||||
| WBC (4–10/uL) | 4,37 ± 1,33 | 5,16 ± 2,34 | 4,82 ± 1,31 | 5,02 ± 1,44 |
| Neu(50-70%) | 37,4 ± 11,39 | 47,28 ± 13,03 | 46,67±12,99 | 44,32 ± 10,15 |
| Neu# (2-7/uL) | 1,41 ± 0,37 | 2,20 ± 0,87 | 2,30 ± 1,66 | 2,29 ± 0,90 |
| Linf(20-40%) | 49,81 ± 10,08 | 39,0 ± 11,42 | 39,9 ± 11,82 | 41,26 ± 10,14 |
| Linf# (0,8-4/uL) | 2,33 ± 0,67 | 1,88 ± 0,68 | 1,99 ± 0,57 | 2,00 ± 0,70 |
| Mon(3-12%) | 9,94 ± 1,39 | 8,43 ± 2,55 | 7,28 ± 2,43 | 12,59 ± 27,99 |
| Mon#(0,12-1,20/uL) | 0,46 ± 0,21 | 0,39 ± 0,12 | 0,36 ± 0,13 | 0,46 ± 0,25 |
| Eos(0,5-5%) | 3,28 ± 2,84 | 5,06 ± 7,44 | 4,61 ± 6,83 | 5,50 ± 6,92 |
| Eos# (0,02-0,5/uL) | 0,17 ± 0,19 | 0,36 ± 0,68 | 0,22 ± 0,31 | 0,26 ± 0,30 |
| Bas(0-1%) | 0,26 ± 0,19 | 0,48 ± 0,42 | 0,49 ± 0,51 | 0,42 ± 0,29 |
| Bas# (0,0-0,1/uL) | 0,01 ± 0,01 | 0,03 ± 0,04 | 0,02 ± 0,03 | 0,03 ± 0,10 |
| IMG#(0,0-100%) | 0,13 ± 0,15 | 0,18 ± 0,21 | 0,17 ± 0,16 | 7,71 ± 43,22 |
| IMG (0,0-999,99/uL) | 0,01 ± 0,01 | 2,45 ± 0,03 | 0,01 ± 0,02 | 0,15 ± 0,72 |
| Erythrogram | ||||
| RBC(3,5-5/uL) | 4,79 ± 0,62 | 3,88 ± 0,52 | 4,06 ± 0,64 | 4,05 ± 0,77 |
| HB(11,7-16,6 g/dl) | 13,43 ± 0,54 | 11,73 ± 2,58 | 11,82 ± 1,75 | 11,41 ± 1,90 |
| Htc(37-54%) | 39,82 ± 1,03 | 34,10 ± 5,7 | 38,33 ± 14,10 | 34,30 ± 5,30 |
| VCM(80-100 fl) | 84,18 ± 10,21 | 88,47 ± 13,09 | 84,73 ± 16,05 | 84,98 ± 14,52 |
| HCM(27-34 pg) | 28,43 ± 4,46 | 30,0 ± 5,41 | 30,24 ± 4,01 | 29,0 ± 4,34 |
| MCHC(32-36 g/dl) | 33,71 ± 1,20 | 33,7 ± 3,14 | 32,92 ± 3,90 | 33,22 ± 1,40 |
| Plachetogram | ||||
| PLT(140-450/uL) | 238 ± 37 | 217 ± 79 | 244 ± 76 | 254 ± 77 |
Note: # is used to differentiate the concentration per microliter from the percentage concentration that is displayed before each parameter.
Table 5: Blood groups and blood count in patients with HIV.
In the Biochemical evaluation, individuals in the group of all blood groups had a mean percentage of normal glucose (between 94 and 100 mg/dL), however, individuals in the ORh+ group had high standard deviation values (SD=37 .71). It was found that the mean value of urea in all blood groups of patients with HIV was within the reference values (13 to 45 mm/dL), however, in patients in the BRh+ and ORh+ groups, the dispersion between the means was greater and that of this so the standard deviations were greater than the means (33.32 mg/dL, SD=63.33, and 26.52 mg/dL, SD=32.32, respectively). We found that the mean value of creatinine was increased in individuals of blood groups, ORh+ (1.42 mg/dL, SD=3.53), ARh+ (1.74 mg/dL, SD=4.05), and BRh+ groups, showed higher (4.84 mg/dL, SD=18.63). It was noticed that patients in the ABRh+ group had a mean cholesterol value (219.0 mg/dL, SD=20.8) higher than the reference values (<190 mg/dL) (Table 6).
| Grupos Sanguíneo | ||||
|---|---|---|---|---|
| Biochemistry | ABRh+ Mean ± SD | ARh+ Mean ± SD | BRh+ Mean ± SD | ORh+ Mean ± SD |
| n=6(4,6%) | n=30(23,1%) | n=33(25,4%) | n=61(46,9%) | |
| Glucose (70-100mg/dL) | 97,53 ± 9,62 | 94,03 ± 12,84 | 97,73 ± 16,63 | 100,0 ± 37,71 |
| Urea (13-45 mg/dL) | 20,31 ± 4,44 | 26,34 ± 13,22 | 33,32 ± 63,33 | 26,52 ± 32,32 |
| Creatinine (0,6-1,3 mg/dL) | 0,94 ± 0,13 | 1,74 ± 4,05 | 4,84 ± 18,63 | 1,42 ± 3,53 |
| TGO(5-40U/L) | 26,54 ± 11,54 | 30,12 ± 16,94 | 36,43 ± 21,82 | 32,23 ± 25,44 |
| TGP(7-56U/L) | 25,46 ± 4,55 | 29,13 ± 18,62 | 32,41 ± 25,24 | 29,15 ± 19,32 |
| HDL((>40 mg/dL) | 63,33 ± 15,86 | 80,14 ± 125,74 | 50,83 ± 14,83 | 55,45 ± 31,91 |
| LDL(<130 mg/dL) | 73,14 ± 34,7 | 94,72 ± 47,9 | 88,74 ± 44,8 | 121,2 ± 168,7 |
| Total Cholesterol(<190 mg/dL) | 219,0 ± 20,8 | 172,0 ± 44,0 | 189,9 ± 42,5 | 177,2 ± 43,0 |
Table 6: Blood groups and Biochemistry examine patients with HIV.
Regarding the age and gender data found in the present study, these seem to be similar to data from a study carried out in Côte d'Ivoire with blood donors to assess the frequency of Hepatitis B and HIV in relation to blood groups (ABO/Rh), showed that among donors the age group from 25 to 44 years being the most represented, with male predominance (sex ratio: 4.79), where the incidence of blood groups A (22.51%), B (22.51%), AB (4.40%), O (49.74 %) and Rh+(97%) and Rh-(3%), although it identified that group O donors were more infected than non-O donors, it did not find a statistical association between ABO blood groups and HIV infection [12]. Data from previous studies developed by our team in individuals with chronic kidney disease, hypertension, and sickle cell anemia, always showed a greater predominance of the ORh+ group in relation to other groups, where the BRh+ and ARh+ groups present values close to each other and higher than the ABRh+ group, however, in children with nephrotic syndrome and sickle cell anemia, the ABR+ group was superior to groups A and B, although, in almost all the studies cited, the Rh- group appears at very low frequencies (less than 3%), the which does not happen in this study.
Regarding education, the data from the present study, seem to corroborate the data from a study carried out in Zambia to assess the impact of education on the possibility of taking an HIV test, which showed that of 6,768 women who declared high school or higher education, 5,426 (80 %) were tested for HIV compared with 1,342 (20%) women who did not (P<0.001), as well as 4,707 (94%) women whose partners had high school or higher education, were tested for HIV compared with 300 (6%) women not tested (P<0.001), where acceptance of HIV testing differed greatly by women's educational level, with greater acceptance observed among women with high school or higher education [AOR 3.8, 95% CI 1.7–8.2; p=0.001] and also noticed that the test was higher in 4,471 (66%) of women living in the urban area, compared to 2,297 (34%) of women in the rural area (P<0.001) [13]. A review study that evaluated blood groups in HIV found that of 50 studies with a total of 3,068,244 where 6,508 were HIV-infected individuals, the analysis showed that blood group AB increased the risk of HIV infection by 19% in comparison with non-AB blood groups (RR=1.19, 95% CI: 1.03-1.39, p=0.02), where pooled estimates for other blood groups did not reach statistical significance and subgroup analysis identified a positive relationship between AB blood group and HIV infection in Asian and non-blood donor populations and populations [14].
The data on complications resulting from HIV in the present study are similar to those found in a study carried out in India, where it was found that the most common complications in an individual with HIV were especially those that affect the respiratory system (56%). , nervous system (26%), skin (15%), and genitals (10%) [15]. The data of associated infections in the present study are similar to those found in a study carried out in South Africa, which identified infections such as Cytomegalovirus (CMV), tuberculosis, hepatitis C and B, Human Papillomavirus (HPV), and others that are still at the center of the problems of health in people with chronic HIV infection and receiving treatment [16].
TCD4, and TCD8, viral load tests allowed for classifying patients into stages of immunity and disease, corroborating a study that concluded that in areas with adequate resources, laboratory measurements of CD4+ T cells and HIV viral load in plasma are commonly used to establish a patient's degree of immunosuppression and rate of immune system destruction and these tools are used to verify a patient's eligibility for treatment and to monitor disease progression, thus with insufficient resources to test cell counts CD4+ T and plasma HIV viral load In many resource-limited settings, including many of the regions hardest hit by the HIV/AIDS epidemic, clinicians must rely on clinical parameters when assessing a patient's disease status [17]. We noticed that 9.2% of the population we studied had given up on treatment and analyzing the data found, leads us to agree with the data of a study developed in China that found that the univariate analysis suggested that the factors associated with evasion of HIV treatment, may include age, area of residence, education, occupation, monthly income, access to the minimum subsidy, HIV transmission route and living conditions, and which factors such as area, monthly income, access to the minimum subsidy livelihoods and referral methods from follow-up institutions account for dropout in the multivariate analysis [18]. Another study also carried out in China, presented slightly different data for adherence, because it showed from 136 individuals, where 13.7% of patients were classified as non-adherent, the expanded regression model revealed additional factors that influence adherence, including coping variables, pain, and numbness in hands and feet, age, stage of illness, fatigue, lack of family support, and twice-daily medication use [19].
Therapeutic alternatives seem to be different for each individual, which leads us to collaborate with the conclusion of a review study that reported that HIV treatment evolved from strenuous regimens with high pill burden, inconvenient dosage, treatment-limiting toxicities, interactions with dietary and drug regimens, incomplete viral suppression, and the emergence of drug resistance to manageable once-daily regimens of one or two pills that can be started early in HIV disease and continued with control of viral replication for much of a person's life. an individual, thus pharmacological advances have been at the frontier of science including facts such as pharmacogenomic discovery and translation (HLA-B&57:01 screening to prevent abacavir hypersensitivity), drug class discovery and formulation (fixed-dose combination, long-acting injectable nano formulations), pharmacokinetic enhancement, and management and delivery decision support tools for complex drug and drug interactions [20,21].
Although for the present study we found several laboratory tests, in many situations this does not happen, therefore, the World Health Organization (WHO) has defined an HIV surveillance system and clinical staging and immunological classification of HIV-related diseases in adults and children, which uses standardized clinical parameters to guide medical decision-making in facilities with no or limited access to laboratory testing that has been shown to be a practical and accurate way of managing HIV-infected patients, with international studies showing agreement between the clinical manifestations included in the WHO staging system and laboratory markers, including CD4 cell count and total lymphocyte count [22,23].
The blood count data in the present study, although presented as an average, do not differ much from those obtained in a study carried out in India, in individuals with HIV hemoglobin ranged from 2.3 g/dl to 19.3 g/dl, RBC ranged from 1.02 million/mm 3 to 6.78 million/mm3, mean corpuscular volume (MCV) ranged from 61.1 to 134.6 fl, mean corpuscular hemoglobin concentration (MCHC) from 25.9 to 36 .2 g/dl, hematocrit ranged from 7% to 57.9%, red blood cell distribution width (RDW-CV) ranged from 12% to 27%, total leukocyte count ranged from 1,400 to 19,100 cells/ mm3, the absolute lymphocyte count ranged from 0.1 to 6,000 cells/mm3 and platelet counts ranging from 18,000/mm3 to 6,70,000/mm3 [24]. A recent study conducted in Ethiopia showed that there was a significant difference in total white blood cell count (WBC), absolute neutrophil count (ANC), red blood cell count (RBC), hemoglobin value, mean cell volume (MCV), mean cellular hemoglobin (MCH), mean cellular hemoglobin concentration (MCHC), erythrocyte distribution width (RDW), platelet count and CD4+ T cells in HIV patients before and after initiation of HAART (P<0.05), This fact may be associated with what was observed in the different blood groups in the present study since BRh+ and ORh+ individuals had more than 50% of their population undergoing treatment for less than 6 years [25].
Regarding biochemical tests, a review study recommended that all patients at the time of HIV diagnosis be evaluated for kidney disease with an estimate of proteinuria and a calculated estimate of renal function (creatinine clearance or glomerular filtration rate [GFR]) (C-III) to allow the caregiver to properly prescribe antiretrovirals that require renal adjustment and if there is no evidence of proteinuria, patients at high risk for developing proteinuric kidney disease (eg, cell count <200 cells/ µL or HIV RNA levels >4000 copies/mL, or those with diabetes mellitus, hypertension, or hepatitis C virus co-infection) should undergo annual screening (B-II) [19]. A study carried out in Nigeria that followed the biochemical changes over 12 months in individuals with HIV, found that the concentration of biochemical parameters; ALP (EC 3.1.3.1; P<0.05), ALT (EC 2.6.1.2; P<0.0001), AST (EC 2.6.1.1; P<0.001), amylase (EC 3.2.1; P>0 .05) and direct bilirubin were significantly increased (upper range) over the months of follow-up, although still within the normal acceptable range, however, they did not observe any notable change in plasma creatinine and urea concentrations when compared to reference values, while that albumin levels decreased non-significantly (P>0.002) when compared to the reference value (Figure 1) [26-28].
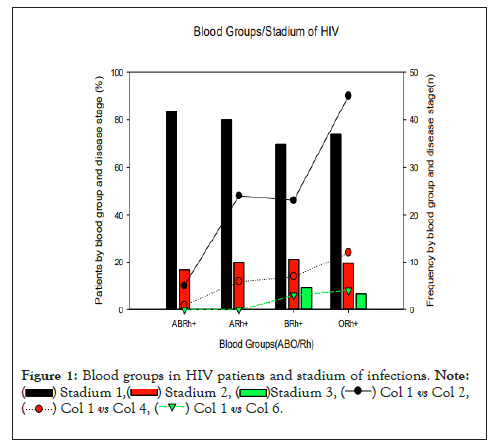
Figure 1: Blood groups in HIV patients and stadium of infections. 
The results indicate that patients with HIV, sociodemographically, are over 30 years old, female, with low education, single and working, most of them have a history of infection by unprotected sexual intercourse, are asymptomatic and with a high viral load, although they receive antiviral treatment, normally does not present very significant changes in the blood count and in the biochemical evaluation.
However, with a high chance of developing renal data, in this group of patients individuals with the ORh+ blood group were the majority, followed by the BRh+ and ARh+ groups, without the presence of Rh- individuals. HIV seems to be common in ORh+ individuals and seems to affect mainly women, young people, people with low education, who have a high viral load, infected for less than 6 years, where changes in the blood count occur moderately in individuals of groups O and A and the biochemical changes in individuals A, B and O.
The authors thank all study participants for agreeing to participate in the research and providing all the information necessary for this study to become a reality. We are grateful to the management and staff of Hospital Josina Machel (Maria Pia), ICISA, CEIP/UPRA, CISA/INIS and CFS for their scientific, technical and institutional support.
The authors declare that there is no conflict of interest.
The authors received no specific funding for this work.
Conceptualization: EMFA, ENMS. Data curation: ENMS, EKC, ATT, SRS, MC, CAPS, CSS, EEV. Formal analysis: ENMS, EKC, SRS, ATT, MC, CAPS, CSS, EEV. Investigation: ENMS, EMFA Project administration: ENMS, EKC, SRS, MC, CAPS, CSS, EEV. Supervision: ENMS. Validation: ENMS, EKC, ATT, SRS, MC, CAPS, CSS, EEV, OFSH. Writing – original draft: ENMS, EKC, CSS. Writing–review and editing: ENMS, EKC, SRS, ATT, MC, CAPS, CSS, EEV, EMFA. All authors have seen and approved the submitted version of this manuscript.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
Citation: Sacomboio ENM, Agostinho EMF, Tchivango AT, Cassinela EK, Rocha Silveira SD, da Costa M, et al. (2022) Sociodemographic Clinical and Blood Group (ABO/Rh) Profile of Angolan Individuals with HIV. J Clin Cell Immunol. 13:675.
Received: 15-Nov-2022, Manuscript No. JCCI-22-20120; Editor assigned: 18-Nov-2022, Pre QC No. JCCI-22-20120 (PQ); Reviewed: 05-Dec-2022, QC No. JCCI-22-20120; Revised: 12-Dec-2022, Manuscript No. JCCI-22-20120 (R); Published: 19-Dec-2022 , DOI: 10.35248/2155-9899.22.13.675
Copyright: © 2022 Sacomboio ENM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.