Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
+44 1300 500008
ISSN: 2157-7544
+44 1300 500008
Research Article - (2015) Volume 6, Issue 2
The solvent extraction of copper(II) from nitrate medium with lauric acid (HL) at 25°C is studied as a function of various parameters: pH, concentration of lauric acid and the nature of solvent. The solvent effects on the extraction of copper(II) using polar and nonpolar solvents are treated. Extracted species differs from solvent to solvent: CuL2 (HL)2 for cyclohexane, dichloromethane, toluene or chloroform and CuL2 for 1-octanol and methyl isobutyl ketone. The extraction constants, percentage extraction (%E) and free energy (ΔG°) are also calculated for different solvents.
Keywords: Solvent extraction; Copper(II); Lauric acid; Solvent effects; Free energy
Solvent extraction system is one of the effective techniques used for separation of metal cations from aqueous solutions [1-8]. It is a suitable method for preventing and protecting environment from pollution. The extraction of copper(II) using carboxylic acids has been a subject of much work research [9-13]. Lauric acid was employed as an extractant for the separation of numerous cations, the composition of extracted species and their extraction constants were reported [14-16]. Ghanadzadeh et al. [17] established that lauric acid extracts capably copper(II) from aqueous solutions and it was shown that in the organic phase dimeric complexes of the (CuR2.HR)2 formula are formed. The solvents have a great importance on the extraction efficiency and distribution ratio due to their polarity [18]. As a consequence, studies of the effect of solvents on the extraction of metal ions have been reported by many researchers [19-23]. Yamada et al. [24] studied the extraction of gallium (III) with decanoic acid in different solvents. They established that the extracted decanoates were more extensively polymerized in the less polar solvent than the more polar solvent. However, Ghebghoub et al. [25] investigated the effect of diluents on the extraction of copper(II) with di(2-ethylhexyl)phosphoric acid. They found that in the extracted species were CuL2 and (CuL2.2HL) in polar and nonpolar diluents respectively. The present paper describes the results obtained from the solvent extraction of copper(II) from nitrate medium by lauric acid in several organic solvents. The scope of the work is to determine the stoichiometries coefficients of the extracted species and their equilibrium constants. In addition, the solvent effects on this extraction system are examined and the interaction with several solvents is interpreted.
Reagents and solutions
Lauric acid (Biochem), Copper nitrate (Biochem) and sodium nitrate (Biochem) were used without further purification. Chloroform, toluene, dichloromethane, cyclohexane, 1-octanol and methyl isobutyl ketone (MIBK), were employed as the organic solvents after washing several times with distilled water. The ionic strength of the aqueous medium was assumed to be unity ([NaNO3]=1M). Aqueous phase: [Cu2+]=1.57 x 10-3 M; [NaNO3]=1M. Organic phase: [HL]=0.01, 0.02 and 0.04M.
Extraction and analytical procedures
Experiments were carried out by shaking equal volumes (25 ml) of both phases in thermostatted vessels. The time required to reach the equilibrium state was 30 min. The pH of the aqueous phase was adjusted by adding the necessary amount of 0.1M NaOH. Then, after the two phases were separated completely by gravity, concentrations of the metal remaining in the aqueous phase were determined photometrically at 820 nm using a Philips UV-VIS SP6-36. The metal ion concentrations in the organic phase were calculated from the difference between the metal ion concentrations in the aqueous phase before and after extraction. All the experiments were carried out at constant temperature T=25°C.
General treatment of extraction equilibrium of Copper (II) with lauric acid
It is well known that carboxylic acids are present as dimeric species in nonpolar solvents such as toluene, hexane or benzene [14,26] and as monomeric species in polar solvents such as: 1-octanol, and 4-methyl- 2-pentanone. [26,27]. The extraction process may be described by the following equilibrium in nonpolar solvents:
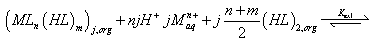 (1)
(1)
Where, equilibrium constant Kex1 is defined as Equation (2) and it can be rewritten as (Equation 3) by using the distribution ratio, D of copper (II).
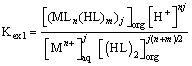 (2)
(2)
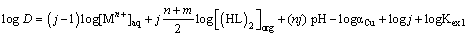 (3)
(3)
Where the distribution coefficient, D, is defined as the ratio between the concentration of metal in organic and aqueous phase.
However, in polar solvents equations (1), (2) and (3) have:
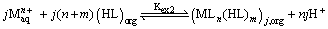 (4)
(4)
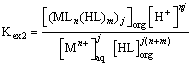 (5)
(5)
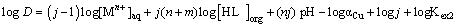 (6)
(6)
Where, the species in the aqueous and organic phases are designated by the subscripts aq and org respectively. Other parameters are defined as:
M=Cu2+, n=cationic charge, m=number of monomeric acids contained in the complex, j=degree of polymerization, (HL)2; (HL)=extractant in dimeric and monomeric form respectively, αCu=the side reaction coefficient allowing for metal complexation in the aqueous phase.
The equilibrium slope method has been used to analyze the experimental data for each extraction system. The stoichiometries of the extracted species were determined on the basis of some equations derived from Equations. (3) and (6). With a predetermined lauric acid concentration in the organic phase, the relationship should yield a straight line of slope j. On the other hand, if only (MLn (HL)m)j is the extractable species in the extraction system and when j and α are equal to unity, the plot of log D versus pH at constant concentration of the extractant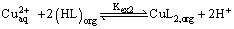 should yield a straight line with a slope of n. In addition, the plot of log D versus log[(HL)]2 and log[HL] at constant pH should give a straight line of slope j(n + m)/2 and j(n + m) respectively, from which the value of m may be calculated. Therefore, the extraction constants can be also determined by intercept with the axis. The percentage extractions (%E) of Copper (II) can be calculated by:
should yield a straight line with a slope of n. In addition, the plot of log D versus log[(HL)]2 and log[HL] at constant pH should give a straight line of slope j(n + m)/2 and j(n + m) respectively, from which the value of m may be calculated. Therefore, the extraction constants can be also determined by intercept with the axis. The percentage extractions (%E) of Copper (II) can be calculated by:
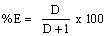 (7)
(7)
Analysis of the extraction equilibrium in nonpolar solvents
In order to investigate stoichiometries of the extracted species in organic phase and to find out which chemical equation applies to the extraction process, a series of Copper(II) extractions were carried out with lauric acid.
First, the degree of polymerization of the extracted copper laurates is given by the slope of the plot (log D + log[Cu+2]aq) versus (log[Cu+2] aq+ 2pH) at a constant [(HR)2]. The results obtained for chloroform, toluene, dichloromethane and cyclohexane are illustrated in Figure 1. It is evident from Figure 1, linear relations are observed with the slope of one (j=1) which indicates that in organic phase monomeric species with lauric acid occur. Second, according to equation (3) the relationships log D versus pH are determined at a constant [(HR)2] in chloroform, toluene, dichloromethane and cyclohexane. The results are shown in Figures 2a-d linear relations are observed with the slope of two (n=2), confirming that the monomeric extracted species in organic phase is CuL2(HL)m (j = αCu =1).
Finally, a study on the effect of lauric acid concentration on Copper(II) extraction enables us to determine the number of lauric acid molecules involved in the extracted species. This effect was investigated by making a series of copper(II) extractions at various [(HR)2] dissolved in chloroform, toluene, dichloromethane and cyclohexane. Concentration of dimer was calculated as [(HL)2]=[HL]/2.
According to equation (3) Plots of log D versus log[(HL)2]orgat constant pH were linear with the slope about of 2, as shown in Figure 3, that is, (2+m)/2=2 or m=2. Consequently, the composition of the extracted species is CuL2(HL)2. A same complex was obtained by Baba et al. [10] in the solvent extraction of copper(II) with 2-butylthiododecanoic acid and 2-bromododecanoic acid in hexane. Therefore, equations (1) and (3) could be rewritten as:
Figure 3: Determination of the number of lauric acid involved in the extracted species at pH=5.2.
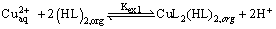 (8)
(8)
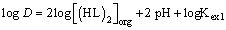 (9)
(9)
Percentage extraction (%E) and the distribution coefficient (D) were calculated to determine the extracting capability of lauric acid diluted in cyclohexane, dichloromethane , toluene and chloroform (Tables 1- 4). The distribution coefficient (D) and extraction percentage (%E) increased with increasing pH. The visible spectra of the loaded organic phase were performed in dichloromethane, toluene chloroform, and cyclohexane (Figure 4). Copper laurates shows an absorbance in the 660-678 nm region which indicated to octahedral geometry coordination of the extracted species [12]. In this study, it was found that two molecules of dimeric lauric acid coordinating with the central copper ion, water molecules would complete the coordination sphere so that the Copper ion could obtain a stable structure. On the basis of this interpretation, the plausible structure of the monomeric copper laurates CuL2 (HL)2 in nonpolar solvents may be written as shown in Figure 5. This structure is similar with that obtained by Adjel [28] on the extraction of copper (II) by capric acid in chloroform.
| pH | [Cu]aqx 10-3 (mol/l) | [Cu]org x 10-3 (mol/l) | D | %E |
|---|---|---|---|---|
| 4.53 | 1.25 | 0.31 | 0.24 | 19.75 |
| 4.60 | 1.18 | 0.38 | 0.32 | 24.77 |
| 4.70 | 1.02 | 0.54 | 0.53 | 34.80 |
| 4.79 | 0.86 | 0.70 | 0.81 | 44.83 |
| 4.84 | 0.78 | 0.78 | 0.99 | 49.84 |
| 4.93 | 0.62 | 0.94 | 1.49 | 59.87 |
| 5.04 | 0.47 | 1.09 | 2.32 | 69.90 |
| 5.15 | 0.31 | 1.25 | 3.98 | 79.93 |
| 5.27 | 0.15 | 1.41 | 8.96 | 89.96 |
Table 1: The distribution coefficient (D) and extraction percentage (%E) for extraction of copper (II) with lauric acid (0.04M) diluted in cyclohexane. [Cu]aq.initial= 1.57x10-3 M.
| pH | [Cu]aqx 10-3 (mol/l) | [Cu]org x 10-3 (mol/l) | D | %E |
|---|---|---|---|---|
| 4.66 | 1.18 | 0.38 | 0.32 | 24.77 |
| 4.71 | 1.10 | 0.46 | 0.42 | 29.78 |
| 4.75 | 1.02 | 0.54 | 0.53 | 34.80 |
| 4.82 | 0.94 | 0.62 | 0.66 | 39.81 |
| 4.86 | 0.86 | 0.70 | 0.81 | 44.83 |
| 4.91 | 0.78 | 0.78 | 0.99 | 49.84 |
| 4.94 | 0.70 | 0.86 | 1.21 | 54.86 |
| 4.99 | 0.62 | 0.94 | 1.49 | 59.87 |
| 5.03 | 0.55 | 1.01 | 1.84 | 64.89 |
| 5.13 | 0.39 | 1.17 | 2.98 | 74.92 |
| 5.24 | 0.23 | 1.33 | 5.64 | 84.95 |
Table 2: The distribution coefficient (D) and extraction percentage (%E) for extraction of copper (II) with lauric acid (0.04M) diluted in dichloromethane. [Cu] aq.initial= 1.57x10-3 M.
| pH | [Cu]aqx 10-3 (mol/l) | [Cu]org x 10-3 (mol/l) | D | %E |
|---|---|---|---|---|
| 4.71 | 1.18 | 0.38 | 0.32 | 24.77 |
| 4.78 | 1.10 | 0.46 | 0.42 | 29.78 |
| 4.87 | 0.94 | 0.62 | 0.66 | 39.81 |
| 4.92 | 0.86 | 0.70 | 0.81 | 44.83 |
| 4.96 | 0.78 | 0.78 | 0.99 | 49.84 |
| 5.01 | 0.70 | 0.86 | 1.21 | 54.86 |
| 5.07 | 0.62 | 0.94 | 1.49 | 59.87 |
| 5.14 | 0.55 | 1.01 | 1.84 | 64.89 |
| 5.19 | 0.47 | 1.09 | 2.32 | 69.90 |
| 5.29 | 0.31 | 1.25 | 3.98 | 79.93 |
| 5.44 | 0.15 | 1.41 | 8.96 | 89.96 |
Table 3: The distribution coefficient (D) and extraction percentage (%E) for extraction of copper (II) with lauric acid (0.04M) diluted in toluene. [Cu]aq.initial= 1.57x10-3 M.
| pH | [Cu]aqx 10-3 (mol/l) | [Cu]org x 10-3 (mol/l) | D | %E |
|---|---|---|---|---|
| 4.8 | 1.18 | 0.38 | 0.32 | 24.77 |
| 4.85 | 1.10 | 0.46 | 0.42 | 29.78 |
| 4.93 | 0.94 | 0.62 | 0.66 | 39.81 |
| 4.97 | 0.86 | 0.70 | 0.81 | 44.83 |
| 5.05 | 0.70 | 0.86 | 1.21 | 54.86 |
| 5.09 | 0.62 | 0.94 | 1.49 | 59.87 |
| 5.14 | 0.55 | 1.01 | 1.84 | 64.89 |
| 5.24 | 0.39 | 1.17 | 2.98 | 74.92 |
| 5.32 | 0.31 | 1.25 | 3.98 | 79.93 |
Table 4: The distribution coefficient (D) and extraction percentage (%E) for extraction of copper (II) with lauric acid (0.04M) diluted in chloroform. [Cu]aq.initial= 1.57x10-3 M.
| pH | [Cu]aqx 10-3(mol/l) | [Cu]org x 10-3(mol/l) | D | %E |
|---|---|---|---|---|
| 4.9 | 1.33 | 0.23 | 0.17 | 14.73 |
| 5.1 | 1.10 | 0.46 | 0.42 | 29.78 |
| 5.2 | 0.94 | 0.62 | 0.66 | 39.81 |
| 5.29 | 0.78 | 0.78 | 0.99 | 49.84 |
| 5.39 | 0.62 | 0.94 | 1.49 | 59.87 |
| 5.5 | 0.47 | 1.09 | 2.32 | 69.90 |
| 5.6 | 0.31 | 1.25 | 3.98 | 79.93 |
| 5.7 | 0.23 | 1.33 | 5.64 | 84.95 |
Table 5: The distribution coefficient (D) and extraction percentage (%E) for extraction of copper (II) with lauric acid (0.04M) diluted in 1-octanol. [Cu]aq.initial= 1.57x10-3 M.
| pH | [Cu]aqx 10-3 (mol/l) | [Cu]org x 10-3 (mol/l) | D | %E |
|---|---|---|---|---|
| 5.33 | 1.023 | 0.54 | 0.53 | 34.80 |
| 5.36 | 0.94 | 0.62 | 0.66 | 39.81 |
| 5.43 | 0.78 | 0.78 | 0.99 | 49.84 |
| 5.54 | 0.62 | 0.94 | 1.49 | 59.87 |
| 5.58 | 0.55 | 1.01 | 1.84 | 64.89 |
| 5.65 | 0.39 | 1.17 | 2.98 | 74.92 |
| 5.79 | 0.23 | 1.33 | 5.64 | 84.95 |
Table 6: The distribution coefficient (D) and extraction percentage (%E) for extraction of copper (II) with lauric acid (0.04M) diluted in MIBK. [Cu]aq.initial= 1.57x10-3M.
| Solvant | logKex | ΔG° (kJ/mol) | Extracted species |
|---|---|---|---|
| Cyclohexane | -10.71 | 61.15 | CuL2(HL)2 |
| Dichloromethane | -11.08 | 63.26 | CuL2(HL)2 |
| Toluene | -11.31 | 64.57 | CuL2(HL)2 |
| Chloroform | -11.39 | 65.03 | CuL2(HL)2 |
| 1-octanol | -11.89 | 67.88 | CuL2 |
| MIBK | -12.14 | 69.31 | CuL2 |
Table 7: The extraction constant and free energy (ΔG°) for extraction of copper (II) with lauric acid diluted in several solvents at T= 298.2 K.
Analysis of the extraction equilibrium in polar solvents
The degree of polymerization of the extracted copper laurates is given by the slope of the plot (log D + log[Cu+2]aq) versus (log[Cu+2] aq + 2pH) at a constant [HR]. The results obtained for 1-octanol and MIBK are illustrated in Figure 6. It is evident from Figure 6, linear relations are observed with the slope of one (j = 1) which indicates that in organic phase monomeric species with lauric acid occur.
According to equation (6), the plots of log D versus pH were determined at a constant [HR] in 1-octanol and MIBK, The results are shown in Figure 7a and Figure 7b, linear relations are observed with the slope of 2 (n=2), confirming that the monomeric extracted species in organic phase is CuL2(HL)m (j=αCu =1). In the other hand, the effect of lauric acid concentration on Copper (II) extraction enables us to determine the number of lauric acid molecules involved in the extracted species. This effect was investigated by making a series of Copper (II) extractions at various [HR] dissolved in 1-octanol and MIBK.
According to equation (6), Plots of log D versus log[HL]org at constant pH were linear with a slope of 2, as shown in Figure 8, that is, 2 + m = 2 or m = 0. Consequently, the composition of the extracted species is CuL2. This result is in accordance with that reported by Yamada et al. [27]. Therefore, equations (4) and (6) could be rewritten as:
Figure 8: Determination of the number of lauric acid involved in the extracted species at pH=5.6.
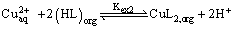 (10)
(10)
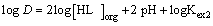 (11)
(11)
Percentage extraction (%E) and the distribution coefficient (D) were calculated to determine the extracting capability of lauric acid diluted in 1-octanol and MIBK (Tables 5 and 6). The distribution coefficient (D) and extraction percentage (%E) increased with increasing pH.
The visible spectra of copper laurates in MIBK and 1-octanol showed an absorbance at 668 and 700 nm respectively (Figure 9). This result indicated to octahedral geometry coordination of the extracted species [27]. In this study, it was found that two molecules of monomeric lauric acid coordinating with the central Copper ion, water and/or solvent molecules coordinate to copper atoms in place of lauric acid molecules. On the basis of this interpretation, the plausible structure of the monomeric copper laurates CuL2 in polar solvents may be written as shown in Figure 10. A similar structure was obtained by Yamada et al. [27] on the extraction of Copper (II) with decanoic acid into 1-octanol.
Analysis of the extraction equilibrium in nonpolar and polar solvents
In order to compare the extraction properties of several solvents, a series of Copper (II) extractions were performed using lauric acid 0.02 M dissolved in toluene, dichloromethane, chloroform, cyclohexane, MIBK and 1-octanol. The results obtained are presented in Figure 11 as plots of log D against pH. The relationships have a linear nature of slopes about to 2. From these results, it was observed that nonpolar solvents showed better performance in Copper (II) extraction than polar solvents. This result is in accordance with that obtained in literature [24]. Also, it is clear that the cyclohexane was as the preferred solvent for copper (II) extraction, may be explained by the total absence of interactions between lauric acid and the solvent. Oukebdane et al. [29] tested the extraction of copper (II) by a mixture of lauric acid and TOPO in different solvents (chloroform, nitromethane and cyclohexane). They observed that cyclohexane as the favored solvent.
On the other hand, the extraction of Copper (II) in dichloromethane is better than in chloroform and toluene, these results can depend on dielectric constants of the solvents. Dichloromethane having high dielectric constants is favored for the extraction of all the metal ions. In addition, the better salvation of the complexes by dichloromethane may be a valuable reason for better extraction [30,31]. The Copper (II) extraction in chloroform is weakest from the stronger interactions between extractant and chloroform in the organic phase, these interactions decreased the activity of lauric acid. However, in polar solvents, 1-octanol and MIBK, it was observed that the distribution coefficient decreased as the polarity of solvents increased. It can be explained that the polar solvents are capable to forming hydrogen bonds with electron donor atoms of lauric acid lead to a reduction in the active concentration of the lauric acid, consequently, poorer extraction of Copper results.
In MIBK, which is a more polar solvent, the extraction proceed rabidly and a lower amount of Copper (II) was extracted than that in 1-octanol, would probably explained by the stronger chemical interaction between MIBK and lauric acid in addition to the solubility of MIBK in aqueous phase. This result is in accordance with that observed by Ghebghoub et al. [25]. While a poor extraction was achieved when using MIBK as solvent on the extraction of Copper (II) by di(2-ethylhexyl)phosphoric acid. Table 7 gives the stoichiometries of the extracted copper species with lauric acid in different solvents and their extraction constants. The log Kex values are higher in nonpolar solvents than in polar solvents. This result can be explained by the extensive solvation of lauric acid in polar solvents [32].
Thermodynamic studies of copper (II) extraction with lauric acid in different solvent
In order to investigate the solvent effects on the thermodynamic parameter of Copper (II) extraction, the free energy (ΔG°) was calculated for different solvents: chloroform, dichloromethane, cyclohexane, toluene, 1-octanol and methyl isobutyl ketone (MIBK) at constant tempetrature T = 298.2 k. The ΔG° is expressed by the following equation [17,33].
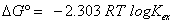 (12)
(12)
Where R is the universal gas constant (8.314 J/mol K), T is the thermodynamic temperature and Kex is the extraction constant. The results obtained are shown in Table 7. It is evident from Table 7, the free energy (ΔG°) increases with the polarity of solvents. In addition, the (ΔG°) value is positive. The positive value of (ΔG°) indicated that the Copper (II) extraction with lauric acid didn’t occurred spontaneously in all solvents examined.
The results obtained in this work have established the feasibility of using lauric acid to remove the heavy metal ions like Copper (II) from aqueous solutions. Lauric acid extracts Copper (II) as a monomeric Copper laurates from nitrate medium according to the following stoichiometric relations.
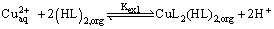 (13)
(13)
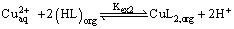 (14)
(14)
The choice of solvent, polar or nonpolar, has a greater effect on the extraction efficiency. The nonpolar solvents showed better performance than the polar solvents on the Copper extraction by lauric acid. The extraction capacity of lauric acid in different solvents decreases in the order: cyclohexane > dichloromethane > toluene ≥ chloroform > 1-octanol > MIBK. The positive value of the free energy (ΔG°) indicated that the copper(II) extraction with lauric acid didn’t occurred spontaneously.