Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Review Article - (2021)Volume 12, Issue 6
Skin rashes in febrile neutropenic immunocompromised patients represent as an important problem in differential diagnosis for their physicians in different specialty. In these patients, rashes may be the first and only evidence of serious and life-threatening infections.
Subjective cognitive decline; Memory complaints; Loneliness; Pain; Mental health; Quality of life
A variety of different cutaneous manifestations in immunocompromised patients and febrile neutropenic patients may be found [1]. In particular patients with genetic conditions that result in decreased neutrophil numbers (e.g., severe congenital neutropenia) or defects in neutrophil function (e.g., chronic granulomatous disease, myeloperoxidase deficiency, leukocyte adhesion deficiency I, and neutrophil specific granule deficiency) are predisposed to S. aureus infections [2].
Neutrophils represent the first-responder phagocytic cells that are recruited from the circulation to the site of infection or inflammation [3]. Acquired neutropenia sometimes results from an infection, can be seen also in cancer patients on chemotherapy, it may be estimated in 50 percent these patients which make them are highly susceptible to infections especially to S. aureus infections [4].
The human immune system is a competent complex system which arises from interactions of divergent immune components that coordinated, then activated in a harmony resulting in adjusted immune response agnist any harmful or infectious agents; viral, bacteria or fungal infections. Humoral and cell-mediated immunity are the main forceful wings of immune defense. Skin is harboring several types of immune cells that participate in innate and adaptive immune responses as an imbricating network of resident immune and non-immune cells that defend and protect the host against harmful infections. Skin starts to serve as a competent barrier to external environment beyond 34 weeks gestational age, while in utero [5]. Antimicrobial peptides (AMPs) and lipids are the main classes of biomolecules that participate in skin defense by disrupting bacterial membranes [6]. The most thoroughly studied AMP families in human skin are the defensins and cathelicidins, which are produced by a variety of cells in the skin such as keratinocytes, fibroblasts, dendritic cells, monocytes, and macrophages, and sweat and sebaceous glands [6]. Skin-resident myeloid cells include Langerhans cells, dermal dendritic cells, macrophages, mast cells, and eosinophils. Neutrophils are rarely found in healthy skin and thus are not “skin-resident cells.” However, neutrophils populate the skin in inflammatory conditions and after a wound. In inflammatory conditions, myeloid cells respond immediately and produce pro-inflammatory mediators that drive the activation of cells in the local vicinity and infiltration of the affected site by peripheral immune cells. Skin myeloid cells also serve as a coordinator between the innate and adaptive immune system [7]. In the last decade new term has been introduced and has been extensively studied, the skin microbiome, this gave us more clarification about the delicate balance between bacteria, skin cells and immune cells. The balanced interaction supports our skin as an immune system and bolsters the body’s defenses against different pathological microorganism. With estimated densities ranging from 104 to 106 microorganisms per square cm [7,8].
Neutrophils the key role cell
Neutrophils are host immune defense cells that are among the first to migrate into the skin in response to invading pathogens. These cells respond to chemotactic signals present at the site of infection, they played major roles in inflammatory and immune conditions through phagocytosis and killing of bacteria via the generation of reactive oxygen intermediates and the release of lytic enzymes stored in granules [9]. In addition to pathogens phagocytosis and subsequent reactive species- and enzyme-dependent pathogen destruction, neutrophils also exert antibacterial activity through Neutrophil Extracellular Traps (NETs), which was first described by Brinkmann et al. [10]. Through this brief review, simplified details which clarify the neutrophils roles in controlling infections, so it is evident that neutropenia is a serious hematological problem in case if it is prolonged and severe. Neutropenia is a critical condition whatever the cause as it can lead to life threatening infection by bacteria, fungi or virus, even if infections originated at skin it might be disseminated to blood causing sepsis, especially in immunocompromised patients underlying either genetic or acquired neutropenia. The most common cause of acquired neutropenia is the administration of chemotherapeutic agents, however there are other conditions also lead to neutrophil depletion or dysfunction, including acute myeloproliferative disorders, myelodysplastic syndromes, aplastic anemia, congenital or cyclic neutropenia, overwhelming sepsis, Felty's syndrome, chronic granulomatous disease, or receipt of certain other drugs including azathioprine, vancomycin, sulfonamides, mycophenolate mofetil, ganciclovir, and tumor necrosis factor (TNF)-alpha inhibitors [11].
During viral infections
The early nonspecific immune responses through natural killer cell activity and interferon limit virus multiplication during the acute phase of virus infections. The later specific immune response includes humoral and cell-mediated responses help to eliminate virus at the end of the acute phase, and subsequently to maintain specific resistance to reinfection. The innate immune responses through macrophages, Natural Killer (NK) cells, and dendritic cells result in subsequent secretion of multiple cytokines and chemokines. One of the most important cytokines is the interferon (IFN), which will activate the antiviral specific immune responses in a fast and effective manner as interferons (IFNs) are the best known for their antiviral properties [12]. The interaction of viral ligands with host receptors including toll-like receptors (TLRs), the cyclic gMP-AMP synthase (cGAS), and the IFN-ɣ-inducible protein 16 (IFI16), which recognize microbial PAMPs activate downstream signaling events that will in turn activate transcription factors, that ultimately regulate the expression of genes implicated in innate and adaptive immunity [13]. Contribution by adaptive immune responses against viral infection informs of generated immune effector cells. T cells directly destroy virus-infected cells or release cytokines, such as tumor necrosis factor (TNF), that damage cells. With some non-cytopathic virus infections, such as HCV and HBV, destruction of infected cells by CD8+ effector T cells is the main cause of damage to the liver [14].
During bacterial infections
A successful immune response to bacterial infection requires a rapid activation of innate immunity, which directs the subsequent development of a productive adaptive immune response [15]. Early secretion of IFN-gamma can be induced by iNKT cells following an encounter with both gram-negative and gram-positive bacteria. Innate receptors that recognize bacterial signals have a crucial role in triggering the antigen presenting cells, which subsequently direct the activation of iNKT cells [16]. The activated antigen presenting cells stimulate the iNKT cells by signaling through toll like receptors (i.e., TLR4, TLR7, and TLR9) leading to the production of IL-12, also other inflammatory cytokines. Study by De Libero and Paget have suggested that TLR signaling through APCs are not only important for cytokine production but also the accumulation of self-lipid antigen for CD1d presentation [17].
During fungal infections
The incidence of fungal infection and clinical fungal-related disease has risen dramatically in the last two decades, which would suggest an increasing pool of susceptible, immunocompromised individuals. These could conceivably include individuals infected with human immunodeficiency virus (HIV), patients with a hematologic or solid cancer, and transplant recipients [18].
The mechanistic aspects of these immune responses (innate or adaptive) vary depending on the fungal species encountered the target organism, and the site of infections. Neutrophils, macrophages, and DCs are all critical to the antifungal response [19]. Upon infection, these innate immune cells are rapidly recruited to sites of infection by virtue of their production of inflammatory cytokines, chemokines, and/or complement units [20]. Immune- regulatory CD4+ T helper cells are of key importance, which can be functionally categorized as one of the five groups: Th1, Th2, Th9, Th17, and TReg cells [21].
Th1 cells can be activated by DCs via TLR signaling, activated in response to the recognition of immutable fungal molecules [22]. Th1 cells can then help to optimize the activation of phagocytes at sites of infections. Th1 cells can also secrete signature pro-inflammatory cytokines such as IFN-γ and TNF-α. Any diminished ability of Th1 cells to mediate inflammatory signaling to phagocytes (such as macrophages) may lead to the decline of the infected patient [23]. Thus, modulating Th1 cells can boost the therapeutic efficacy of antifungal agents. Th2 cells, activated by IL-4 and IL-13, generate cytokines including IL-5 that can limit the Th1 response, as well as activating M2 macrophages, which are harmful to patients with severe fungal infections and fungal-related allergic responses [22].
Skin and soft tissue infections in immunocompromised patients (HIV and Non HIV-infected persons)
Cutaneous infections are common in immunocompromised patients with neutropenia which predisposes fungal, bacterial and viral infections. Skin and Soft Tissue Infections (SSTIs) encompass a variety of conditions; in immunocompromised hosts, and they pose a major diagnostic challenge. In a previous study, hospitalized febrile neutropenic patients with hematologic malignancy, aplastic anemia, or bone marrow transplantation were included [24]. Patient files were screened retrospectively and patients with skin lesions during febrile neutropenic episodes were selected. Patients with febrile neutropenia were initiated an anti-pseudomonal beta-lactam therapy according to IDSA guidelines. Skin lesions of these patients during febrile neutropenic episode, consulted and evaluated with infectious disease and dermatology specialists. Skin and soft tissue infections (SSTIs) encompass a variety of conditions account for a large percentage of infections requiring hospitalization, and are associated with multiple variable of skin rash in febrile neutropenic patients including skin and (SSTIs) [25].
Viral, fungal and bacterial infections as well as inflammatory dermatoses have all been reported with increased frequency in association with HIV infection [26]. In non-HIV immunocompromised hosts, including patients with solid organ transplants, stem cell transplants, solid tumors, hematologic malignancies, and receiving chronic immunosuppressive therapy for inflammatory disorders, gram-negative bacteria like Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii are usually associated especially in cancer patients. At the same time, inadequate empirical/therapeutic therapy with antibiotics exposes these patients to increased risk of adverse outcome, especially in neutropenic bacteremic patients suffering from MDR infections [27].
Case 1
Male patient 42 years old, a known case of acute myeloid leukemia on chemotherapy in KCC, presented to our center as consultation from his oncologist due to erupting skin lesions. On examination patient was febrile temp. 38.3°C, regard skin there were multiple tender polymorphous erythematous heamorreghic papules and pustules with necrotic ulcerations on axillae, groin and gluteal areas. CBC showed severe neutropenia, skin culture done from lesions which showed Pseudomonas aeruginosa (Figure 1).
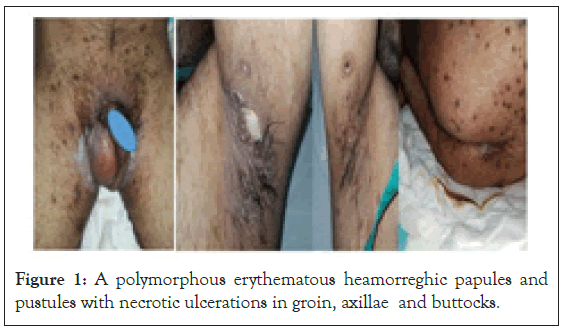
Figure 1: A polymorphous erythematous heamorreghic papules and pustules with necrotic ulcerations in groin, axillae and buttocks.
Case 2
A 15 months old boy infant, a known case of acute leukemia on chemotherapy. A dermatology consultation was sent for developing skin lesions, on examination patient was febrile and has 3 dusky red papules with central pustules and two clear vesicles on a faint erythematous base at middle side of left thigh. C.B.C of patient showed severe neutropenia. Skin culture of the lesions showed S. aureus (Figure 2).
Figure 2: A dusky red papules with central pustules and two clear vesicles on a faint erythematous base.
Case 3
A three years old boy, a known case of leukemia on chemotherapy. He has admitted in pediatric oncology department and his physician had asked for dermatology consultation, as he developed skin lesions on his dorsum of right hand. On examination there were grouped vesicles which coalesced together to form bulla with a necrotic center and surrounding erythema on dorsum of right hand, same lesions on right palm with few scattered erythematous macules. Ecthyma gangrenosum was put as provisional diagnosis. Tissue culture was done which revealed Aspergillus spp (Figure 3).
Figure 3: Grouped vesicles forming bulla on right dorsum of hand and palm with few scattered erythematous macules (blue arrows).
Case 4
Female patient 52 years old was admitted in IDH as a case of histoplasmosis. Her physician had sent a dermatology consultation for further investigations and proper management through a multidisciplinary team management. On examination, patient was febrile and showed three deep submandibular ulcers on right side of the neck for 3 months duration, multiple crusted nodules on the right cheek of one month duration, multiple scalp swellings and palpable submandibular LNs. Biopsy from submandibular LN was done in other dermatology department hospital and showed fungal infection (histoplasmosis) before transferred to IDH. The previous biopsy was reviewed in KCC, it was suspicious of Hodgkin's lymphoma, a recommended LN excisional biopsy to confirm. C.T was done for the multiple scalp swellings and brain which showed TB osteomyelitis with small epidural collections (Figure 4).
Figure 4: Three deep submandibular ulcers on right side of the neck duration and multiple crusted nodules the right cheek.
Our work for this case: D.Dx. for skin lesions were
• Histoplasmosis vs. Scrofuloderma
• Skin biopsies from skin ulcer done for H&E histopathological examination, for AFB, TB culture and TB PCR, tissue culture for bacterial and fungal examination
• Skin swab for C and S
• Blood sample for D Glucan and Galactomannan to exclude invasive fungal infections.
• Neurosurgery consultation to assess scalp swelling with pus collection to exclude deep involvement of subcutaneous and osteomylitis at these areas
• Treatment options were discussed with the treating physician and patient was started on Ambisone and anti TB treatment, topical conservative treatment for ulcers.
Follow up results case 4: Skin biopsy showed suppurative granulomas and neutrophilic micro-abscess with multinucleated giant cells as seen in histopathology images with detailed features (Figures 5-7) which suggestive fungal or bacterial infections.
Figure 5: On low power a dense inflammatory infiltrates involving superficial and deep dermis were seen, with edema in the upper part of the dermis.
Figure 6: A closer view with a higher power shows suppurative granulomas involve the entire dermis, this granulomatous tissue reaction formed by multiple multinucleated giant cells, with dense histiocytes and neutrophilic micro-abscesses.
Figure 7: This is showing neutrophilic micro-abscess and multinucleated giant cells.
PAS and sliver stains (Figure 8) were done to confirm the diagnosis of Hisoplasmosis deep fungal infection. PAS stain was not illustrative, but there were silver stained small round, dark- hyphae and yeast in the papillary dermis demonstrating fungus. Tissues culture for TB was negative, pus swab from scalp was positive for Histoplasmosis species. HIV tests were Negative.
Figure 8: PAS and silver stains were done and it was not illustrative, but there are silver stained small round, dark-hyphae, yeast in the papillary dermis demonstrating fungus.
Follow up case 4: Patient was regularly followed up in coordination with her treating physician in IDH. The adminasrated treatment were anti-tuberculosis drugs, Ambisome (amphotericin B deoxycholate) injection and conservative topical treatment for skin ulceration, after 10 days patient became afebrile and skin ulcers showed signs of healing and also veruccous lesions improved (Figure 4 lower image).
Case 5
A female child 1 year and 6 months admitted to pediatric surgery department for intestinal obstruction, a C.T was done and showed rectal duplication. Patient had recent +ve PCR swab for COVID 19 infection month ago, she has developed skin lesions on her feet and upper back then gradually spread to other sites one week eariler the swab demonstrated the diagnosis of COVID 19 infection. On examination, patient was feverish (temp 37.8°C) otherwise she was vitaly stable. Skin examination showed flesh colored dome shaped papulovesiclar lesions, some of them are umblicated and some crusted as grouped lesions on chest, and natal cleft, others solitary scattered ones on axillae, fingers, feet and face.
DDx. molluscum contagiosum vs. molluscum like lesions (histoplasmosis and aspergillosis): Skin biopsy was taken after a signed consent taken from her parent, viral serology (including herpes, varicella, coxsackie and parvovirus), Serology to exclude opportunistic fungal infections (histoplasmosis and aspergillosis). PCR was done to rule out COVID 19 reinfection.
Follow up after one week: Skin lesions on the right cheek as well as the ulcers are improving well. Patient became afebrile. Lab tests were normal with slight neutropenia in CBC. Patient improved after taking oral acyclovir after discussion with his pedatrician, while the investigations were still pending. Outcome of investigations was done. Skin biopsy showed herpetic viral infection viral serology also unfortunately has showed HIV positively reactive for child and her parents (Figure 9).
Figure 9: Showed flesh colored dome shaped papulovesiclar lesions, some of them are umblicated and some crusted as grouped lesions on chest, and natal cleft, others solitary scattered ones on axillae, fingers, feet and face.
Case 6
A 28 years old single gentleman cigarette smoker (20 cigarettes per day) who asked medical advice in February 2021 in our center, that he complains of skin eruptions three weeks ago inform of wide- spread non-pruritic, painless, multiple, variable-sized, ulcerated and crusted skin nodules on the face, trunk, forearms legs and scalp. He also complained of intermittent fever, arthralgia (small joints of hands) left eye redness associated with blurred vision. Patient has had history of camping in the desert and contact with animals, otherwise no medical history of any chronic illness neither hospital admission nor surgical operation. On examination there are ulcerated papules with hemorrhagic, thick crusts erythematous, ulcerative nodules with central necrosis surrounded by erythema and left eye conjunctivitis. Patient also had oral and genital ulcers, no palpable lymph nodes. Our differential diagnoses were cryptococcosis, deep fungal infection, cutanous Leishmaniasis, a typical mycobacteria, disseminated or for pyoderma gangrenosum (Figure 10).
Figure 10: Multiple ulcerated papules and nodules covered by thick crusts with surrounding erthyma on the upper back, scalp well demarcated areas of alopecia on scalp.
Sexual history: Patient had history of unprotected sex with multiple partners, so STDs need to be ruled out. Patient was referred to IDH for admission and full work up screening of any STDs or opportunistic infections.
Full blood tests were done, C.B.C, L.F.T, R.F.T and lipid profile which were all normal except, very high E.S.R=107/hour. HIV screening was positive, a new diagnosis of HIV. Serological tests for syphilis had showed very high titers VDRL=1: 64 and TPHA=1: 81920, while hepatitis virus screening, CMV IgM, EBV IgM were negative. Chlamydia trachomatis, Gonorrhea, Toxoplasma, Mycobacteria blood culture Cryptococcus antigen were negative. T-spot TB was non- reactive negative, HLA-B57.
HIV viral load and related investigations were: CD4 count=140, HIV viral load=69700 copies/ml, HIV genotype: HIV-1 subtype CRF06-epx, HLA-B57: negative= Skin biopsy was done for H&E and special stain.
Skin biopsy result Special Stains: Gram stain, acid-fast bacilli and periodic acid-Schiff stains all were negative. Cultures; bacterial, fungal were negative, skin swab showed no growth and leishmania smear was negative. One from upper back, which showed psoriasiform epidermal hyperplasia with long thin rete ridges, Interface dermatitis with vacoular degeneration of basal layer with Superficial and deep dermal perivascular and lymphocytic infiltration and abundant plasma cells and neutrophils in association with endothelial swelling. Final diagnosis; based on biopsy findings and serological tests is Secondary syphilis; Lues Maligna type (Figure 11).
Figure 11: Biopsy Site: a) upper back, showed Psoriasis form epidermal hyperplasia with long thin rete ridges, Interface dermatitis with vacoular degeneration of basal layer Superficial and deep dermal perivascular and lymphocytic infiltration Abundant plasma cells and neutrophils, Endothelial swelling, b) a higher power view of previous finding, c) Dermal perivascular and lymphocytic infiltration and abundant plasma cells and neutrophils.
Patient has started on benzathine penicillin 2.4 million units deep intramuscular weekly for 3 weeks, while for HIV a Biktarvy- Bictegravir/emtricitabine/tenofovir alafenamide and spetrin were given and viganox eye lobrex ointment after ophthalmologist coscultation after exclusion of ocular syphilis. Skin lesions healed (Figure 12).
Figure 12: Back, face and scalp lesions healed and a scar in place of skin biopsy.
Risk of SJS/TEN or DRESS when administering abacavir RA. About two months later (on May 2021) patient skin lesions completely healed after starting treatment figure, also Lab work up CD4+: 236 (versus 120 in March) HIV viral load: 32 copies/ml (versus 89453 in March).
Skin and Soft Tissue Infections (SSTIs) in immunocompromised hosts caused by bacteria, fungi, viruses, mycobacteria or even protozoa could resemblance a variable skin presentations, and mostly with substantial association with serious morbidity or even mortality [28]. Diagnosis skin and SSTIs are challenging, so clinical dermatological assessment and well-planned strategy for prompt diagnosis and consequently appropriate management, a list of different including should be initially performed to identify the investigating lines should be done, a blood and tissue cultures, skin biopsy with histology, further more specific microbiological, analysis with special stains, molecular techniques, and antigen-detection methodologies all are mandatory. All available methodologies in our center were done for imperative broad differential diagnosis [29]. A bona fide-out-comes were obtained for our presented cases with. Skin and SSTIs can be caused by diverse microorganisms- most commonly involve microbial invasion of the layers of the skin and underlying soft tissues, ranging from mild infections such as impetigo or ecthyma, to serious, life-threatening infections, such as necrotizing fasciitis [30]. Our case 1 and case 2 caused by gram negative and gram positive bacteria, Pseudomonas aeruginosa and S. aureus respectively in immunocompromised case 1 and case 2 who have acute leukemia on chemotherapy, their CBC showed severe neutropenia. Case 1 has showed skin lesions inform of polymorphous erythematous haemorrhagic papules and pustules with necrotic ulcerations and clear vesicles
Case 2 has showed skin lesions inform of dusky red papules with central pustule. Both case 1 and case 2 have diagnosed bacterial soft tissue infection in immunocompromised patients, classically reported to occur with Pseudomonas aeruginosa gram negative bacteria or other species such as Aeromonas spp, and gram positive bacteria S. aureus. A dramatic increase in community-associated methicillin- resistant is more recently have been documented and affect individuals, even those without prior risk factors [31]. Patients with acute leukemia are at increased risk of developing infections both as a result of the leukemia and chemotherapy drugs [32], Gram- positive bacteria are isolated most often (˜75–80% of the time) with organisms colonizing the skin e.g., staphylococcus species, bacillus species, corynebacterium species) being predominant [33]. Case 3 is skin infection by Aspergillus spp, inform of bulla with a center necrotic. Invasive mold infections, due primarily to inform of spergillus species, are the most frequent cause of serious, often life-threatening infections in patients with neutropenia that [34].
Case 3 is skin infection by Aspergillus spp in an immunocompromised patient as our case known leukemia on chemotherapy, diagnosed according to tissue culture and was treated properly with his physician. Treatment for aspergillosis includes amphotericin B and its lipid formulations, itraconazole, voriconazole, posaconazole, and the echinocandins [35].
The immunosuppression caused by many factors such as malnutrition, autoimmune disease, HIV infection or immunosuppressive drugs has contributed to the increased incidence of tuberculosis (TB) in the world, case 4 patient is HIV negative and she has T.B osteomyelitis with small epidural collections, and she developed very aggressive deep submandibular ulcers, which diagnosed as cutaneous histoplasmosis via skin biopsy with special stain processing. Although co-occurrence of tuberculosis with histoplasmosis occur in advanced HIV patient with a higher mortality rates [36], but histoplasmosis may occur in populations at-risk include persons exposed to large inoculum, immunocompromised patients, extremes of age and underlying emphysema and rather more in aged smokers all are predisposed to chronic pulmonary disease, which is frequent in endemic areas, with common asymptomatic cases [37]. Histoplasmosis is endemic in the central and eastern states of the USA, around the Ohio and Mississippi river valley, but also in parts of central and South America, southern and sub-Saharan Africa, India, China, and Southeast Asia [38]. Case 4 has been started on proper treatment protocol by her physician in IDH, and she has improved regard her skin lesions Figure 4 lower image. The previous four presented cases (1-4) all were reported in non-HIV immunocompromised hosts, immunodeficiency was due to leukemia and immunosupressive chemotherapy [29], however case 4 has cutaneous histoplasmosis associated systemic T.B, the opportunistic infections in case 4 may be due her racial background [39]. Cutaneous infections are extremely common among patients with the human immunodeficiency virus (HIV), and their incidence increase with deteriorating immune function [40]. The mucocutaneous viral infections may represent the first clinical sign of HIV infection [41]. Case 5 was not known to be HIV patient but the proper dermatological assessment has settled diagnosis of dissminated herpetic type 1 skin infection infection, so HIV viral serology screening was done for patient and her parents which have showed unfortunately HIV positively reactive in all. In the majority of immunocompromised hosts, the HSV infection is not primary but rather reactivation of latent HSV, Other variant of atypical herpes simplex virus infection is deep linear fissures in the skin folds (inframammary, infra-abdominal, inguinal, or vulvar) termed the “knife-cut sign”, same seen in case 5 [41], a case of disseminated cutaneous herpes simplex virus-1 reported as the first manifestation of human immunodeficiency virus Infection [42]. Our last case 6 Lues maligna also was not known as HIV and through our proper strategy of management diagnosis of HIV has been detected. Lues maligna is a great impostor in an HIV-infected male [43]. Lues maligna (LM) is a rare dermatologic manifestation of Treponema pallidum, case 6 had been presented by a wide-spread non-pruritic, painless, multiple, variable-sized, ulcerated and crusted skin nodules on the face, trunk, forearms legs and even scalp. This clinical presentation is a diagnostic challenge and can mimic other diseases such as fungal, mycobacterial and herpetic infections, as well as, pityriasis lichenoides et varioloformis acuta [44]. The risk behaviors associated with acquiring syphilis is increasing the likelihood of acquiring HIV [45].
Clinicians must be aware of this rare cutaneous manifestation of a common sexually transmitted illness (STI) in the differential diagnosis, especially in those populations who are at risk, like our case who has multiple sexual partners. Skin manifestations was a common and striking feature of underlying HIV-infection, the presence of skin manifestations which are atypical in morphology ,number or anatomical sites should be regarded as a strong indication for HIV testing and especially as it may reflect advanced immunosuppression The hallmark of LM is the dermatological finding of multiple pleomorphic round-to-oval papules, papulopustules or nodules with ulceration and brown lamellar crusted lesions disseminated over the trunk and extremities [46].
Early recognition and diagnosis can have significant clinical implications, as skin manifestations may be the first indication of underlying hematologic malignancy or HIV and reflect the immune status and stage of disease. It is important to recognize the characteristic skin lesions associated with each specific infection.
Given that the pool of immunocompromised individuals is rapidly expanding, there would appear to be in need of proper diagnostic strategy and alert clinicians. Furthermore novel analytic techniques capable of detecting infecting agents are supporting the scientific research. Upon accurate diagnosis of skin infections, safe and efficient therapies were found especially for immunocompromised hosts. In the first and the other human immune system functions of both innate and adaptive immunity are the two main branches of immunity acting the art of war against harmful invaders to our body and our skin.
Citation: Almasry I, Lazarevic V, Alraqi A, Karam TM, Tawfeek WW, Alraini A, et al. (2021) Some Cutaneous Manifestations in Febrile Neutropenic Immunocompromised Patients, Evidence-Based Case: Review Article. J Clin Exp Dermatol Res. 12:589.
Received: 08-Dec-2020 Accepted: 21-Dec-2021 Published: 28-Dec-2021
Copyright: © 2021 Almasry I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.