Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2023)Volume 14, Issue 2
The aim of this paper is to describe a modified technique of ab-interno implantation of the XEN gel stent. At the beginning of the surgery, the injection of 0.1 mL of air, and then of 0.1 mL of Balanced Salt Solution (BSS) in the subconjunctival space allows you to obtain a mechanical dissection between the conjunctiva and the Tenon’s capsule, transforming a virtual space in a real one. 23 eyes of 22 patients underwent the implantation of a XEN stent gel through the sparkling XEN technique. We retrospectively analyze the results with a reduction of the Intraocular Pressure (IOP) from an average of 16.9 ± 4.6 mmHg preoperatively to at 13.3 ± 1.4 mmHg at month 12. Needling was performed only in one case (4.34%). Two patients had hypotony (IOP<6 mmHg) at post-operative day 1, which self-solved, but we didn’t register any case of hypotony maculopathy and choroidal detachment. The gently dissection of the subconjunctival space performed with air and BSS makes the implantation easier and more predictable, consequently improving the surgical results, and at the same time preserve the tissue from excessive intraoperative handling.
Glaucoma; Bleb management; Valve; Micro-invasive filtering surgery
Glaucoma is a major public health issue. It represents a leading cause of irreversible blindness worldwide [1]. Intraocular Pressure (IOP) represents a primary and modifiable risk factor for the onset and progression of disease [2]. Whenever medical and laser treatments are ineffective to obtain an optimal reduction of IOP (target IOP), surgery is recommended. There are lots of different procedures, but they all acts lowering intraocular pressure, consequently preventing optic nerve damages and future vision loss. Trabeculectomy and glaucoma drainage devices (e.g., Molteno®, Baerveldt® and Paul® implants, Ahmed® glaucoma valve) are the conventional filtering surgery. Recently, new devices are emerging thanks to their safety profile and less invasiveness, termed minimally- or Micro-Invasive Glaucoma Surgery (MIGS). MIGS devices have different mechanisms of action based on the site of anatomical intervention [3].
The XEN gel stent (Abbive, Inc.) is a drainage device developed to lower intraocular pressure of patients affected by Primary Angle Closure Glaucoma (PACG). It shunts the humor aqueous from the anterior chamber to the subconjunctival space, thus creating a new humor outflow pathway. Despite, this is associated to potentially bleb-related complications, these MIGS can potentially achieve efficacy approaching that of trabeculectomy [4].
However, in glaucomatous patients you can find an inflamed conjunctiva, because of the prolonged topical anti-glaucomatous therapy and previous surgeries, and you can also find adhesions between the conjunctiva and the Tenon’s capsule [5]. The outcomes of glaucoma surgeries are limited by postoperative fibrosis, and most commonly this is due to bleb scarring.
The correct implant of the XEN gel stent is the most important step for a successful surgery and long-lasting Intraocular Pressure (IOP) control. It depends on the exact positioning of the stent in the subconjunctival space, above Tenon’s capsule. The conjunctiva is generally loosely attached to the Tenon’s capsule, creating a virtual space. If the positioning is incorrect the device could be deeper within Tenon’s or embedded into this tissue and it is a well-known risk factor for failure. Fibrosis may occur with higher risk of obstruction [6].
In this manuscript we have presented a modified technique of ab-interno XEN gel stent implantation for Primary Open Angle Glaucoma (POAG), named sparkling XEN, where a mechanical dissection of the subconjunctival space is performed in the early phase of the surgical procedure, and our promising results.
All surgeries were performed by one of the authors (Fabrizio Franco) between January and December 2021 at the Eye Clinic, Department of Neuromuscular and Sense Organs, Careggi University Hospital (Florence, Italy). After disinfection of periocular skin and conjunctival fornix with povidone iodine 10% and 5% respectively, a topical anesthesia with 2.5 ml of lidocaine 2% is performed. Then we proceed to sparkling dissection with air and BSS. The first step is to identify the area of the future bleb, usually in the supero-nasal quadrant; then with a dermographic pen and a compass we mark the area at 3.0 mm from the limbus where the XEN gel stent will come out as shown in Figure 1A. Afterwards we perform the dissection. We enter the conjunctiva at 12 o’clock at 3 mm from the limbus with a 27-gauge needle on an insulin syringe and, positioning the bevel up very superficially beneath the conjunctiva avoiding perforating the Tenon’s capsule, and then we arrive in the marked target area directing the tip of the needle posteriorly as shown in Figure 1B. At this point 0.1 mL of air is injected; if the pneumo- dissection has been successful, bubbles will form in subconjunctival space as shown in Figure 1C. Then, 0.1 mL of BSS is injected as shown in Figure 1D. Once the conjunctiva is well separated from Tenon’s capsule, we perform a gentle intraoperative needling the break residual adherence between conjunctiva and Tenon’s capsule, then we implant the XEN gel stent with the traditional ab-interno approach as shown in Figure 1E. A cohesive viscoelastic (Healon GV Pro, Johnson and Johnson) is injected to fill completely the Anterior Chamber (AC) through a clear corneal incision. The disposable injector enters the AC through a main corneal incision in the infero-temporal sector, upon staining the stent with a blue dye (Trypan blue) and is directed toward the supero- nasal angle. An indirect gonio lens is used to watch the angle (a direct view gonio lens can also be used). The correct placement is just anterior to the pigmented trabecular meshwork. Once the needle tip is in the correct place, it is pushed forward through the sclera coming out in the subconjunctival space 3.0 mm away from the limbus. The injector is actioned, and the needle retracts into the sleeve. The device correctly positioned is 1 mm in the AC, 2 mm in the scleral tunnel and 3 mm in the subconjunctival space; the stent is well visible under the conjunctiva since it has been previously colored, it must be linear and with the tip mobile as shown in Figure 1F. The right placement in the angle is verified using a gonio mirror. The viscoelastic is removed from the anterior chamber with balanced salt solutions. Finally, we perform a subconjunctival injection of 0.1 mL of Mitomycin C (MMC) 0.02% in the bleb (Figures 1A-1F).
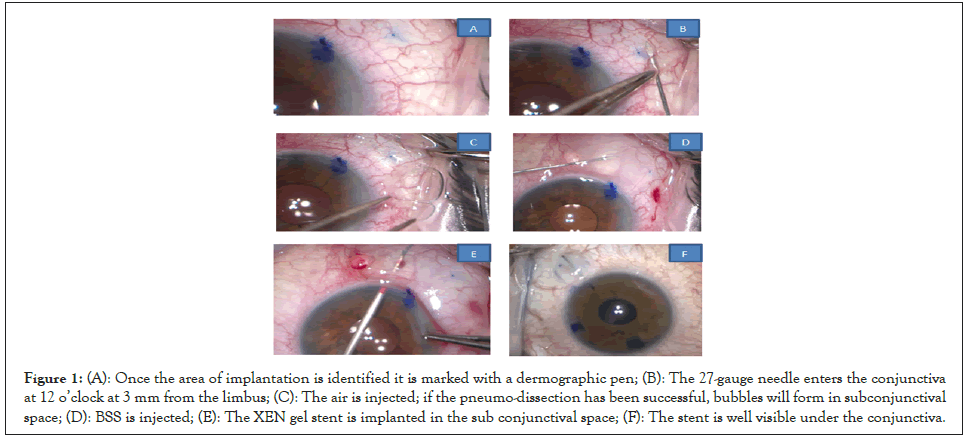
Figure 1: (A): Once the area of implantation is identified it is marked with a dermographic pen; (B): The 27-gauge needle enters the conjunctiva at 12 o’clock at 3 mm from the limbus; (C): The air is injected; if the pneumo-dissection has been successful, bubbles will form in subconjunctival space; (D): BSS is injected; (E): The XEN gel stent is implanted in the sub conjunctival space; (F): The stent is well visible under the conjunctiva.
Patients are instructed to discontinue all glaucoma medications on the day of the surgery; follow-up visits are performed at the postoperative day 1, every week for the first month, at month 3, month 6 and month 12, and included a general assessment and IOP measurements (Goldmann tonometry). Assessments of bleb morphology using Anterior Segment-Optical Coherence Tomography (AS-OCT) images (MS39 CSO Scandicci) were performed at post-operative visits at day 7 and month 1 and whenever required. Post-operative therapy includes antibiotic prophylaxis for 1 week and steroids, tapered in 3 months.
We report our data on 23 eyes of 22 patients; all had diagnosis of POAG, with exception of one case with diagnosis of Post Traumatic Glaucoma (PTG). Five eyes (21.7%) were already pseudophakic, 18 eyes were phakic and underwent simultaneous XEN implantation and cataract surgery. Table 1 summarizes the patients characteristics here in described. The preoperative number of anti-glaucomatous molecules was on average 2.4 ± 1.2 (Table 1).
| Variable | Overall(n=22) |
|---|---|
| Age (mean value ± SD) | 76.4 ± 9.3 |
| Sex (Male/female) | 17/5 |
| Type of glaucoma | |
| POAG | 21(95.5%) |
| PTG | 1(4.5%) |
| Previous surgery | |
| Phacoemulsification+intraocular lens implantation | 21.70% |
| Number of antiglaucoma medications | 2.4 ± 1.2 |
Note: SD: Standard Deviation; POAG: Primary Open Angle Glaucoma; PTG: Post Traumatic Glaucoma
Table 1: Baseline patients’ characteristics.
We obtained a good reduction of the IOP from an average of 16.9 ± 4.6 mmHg preoperatively to 10.3 ± 3.2 mmHg 7 days after the surgery. At 1 month post-surgery average IOP was 11.3 ± 2.2 mmHg, at 3 months it was 13.1 ± 2.9 mmHg, at 6 months 12.7 ± 1.5 mmHg and at 12 months 13.4 ± 1.4 mmHg (Table 2).
| Patient | IOP pre-operation | IOP Day 1 | IOP Day 7 | IOP Month 1 | IOP Month 3 | IOP Month 6 | IOP Month 12 |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 7 | 12 | 11 | 12 | 13 | 15 |
| 2 | 19 | 4 | 7 | 9 | 12 | 13 | 12 |
| 3 | 16 | 9 | 10 | 10 | 12 | 13 | 13 |
| 4 | 16 | 8 | 8 | 8 | 10 | 10 | 11 |
| 5 | 24 | 10 | 10 | 12 | 15 | 15 | 15 |
| 6 | 15 | 8 | 8 | 10 | 12 | 14 | 13 |
| 7 | 17 | 17 | 20 | 14 | 13 | 12 | 13 |
| 8 | 11 | 11 | 11 | 12 | 12 | 13 | 14 |
| 9 | 14 | 12 | 13 | 14 | 12 | 13 | 14 |
| 10 | 27 | 10 | 11 | 12 | 12 | 13 | 12 |
| 11 | 28 | 8 | 8 | 8 | 20 | 12 | 13 |
| 12 | 12 | 8 | 8 | 9 | 11 | 13 | 12 |
| 13 | 14 | 15 | 16 | 12 | 22 | 11 | 14 |
| 14 | 16 | 13 | 13 | 15 | 14 | 14 | 16 |
| 15 | 18 | 8 | 9 | 9 | 12 | 12 | 12 |
| 16 | 18 | 12 | 10 | 12 | 12 | 12 | 13 |
| 17 RE | 12 | 8 | 8 | 13 | 14 | 13 | 14 |
| 17 LE | 12 | 8 | 11 | 11 | 11 | 12 | 13 |
| 18 | 12 | 10 | 10 | 15 | 16 | 16 | 16 |
| 19 | 21 | 4 | 4 | 8 | 9 | 9 | 12 |
| 20 | 18 | 9 | 9 | 11 | 11 | 12 | 12 |
| 21 | 16 | 10 | 11 | 12 | 12 | 13 | 13 |
| 22 | 17 | 10 | 11 | 13 | 15 | 14 | 15 |
| Mean ± SD | 16.9 ± 4.6 | 9.5 ± 3.0 | 10.3 ± 3.2 | 11.3 ± 2.2 | 13.1 ± 2.9 | 12.7 ± 1.5 | 13.4 ± 1.4 |
Note: IOP: Intraocular Pressure; SD: Standard Deviation; RE: Right Eye; LE: Left Eye
Table 2: Intraocular pressure reduction from baseline.
The probability of complete success, meant as IOP ≤ 18 mmHg at month 12 without IOP medications, surgically revising the bleb or reoperation, was of 87%. The probability of partial success (IOP ≤ 18 mmHg at month 12 with the use of medications or following bleb revision) was 13% and the probability of or unsuccess (IOP>18 mmHg with the need for reoperation) was 0%. Needling with 5-Fluorouracil (5-FU) was performed in one case (4.34%) at month 1, and it was resolutive. None needed reintervention.
We had two cases (8.7%) of hypotony (IOP<6 mmHg) at post- operative day 1. One self-solved within the first week, the other one persisted at post-operative day 7 and subsequently solved. No cases needed intervention. None had hypotony maculopathy and choroidal detachment.
The best correct visual acuity improved after the combined phacoemulsification-XEN surgery and remained unchanged in the stand-alone procedure as shown in Table 3. During the follow up we added anti-glaucomatous therapy in three eyes (13%). One of them had controlled IOP with one molecule, two required two different molecules. Post-operative number of anti-glaucomatous molecules was on average 0.2 ± 0.6 (Table 3 and Figure 2).
| Variable | Overall(n=23) |
|---|---|
| Needling | 1(4.34%) |
| Re-intervention | 0 |
| Post-operative number of anti-glaucomatous molecules (Mean ± SD) | 0.2 ± 0.6 |
Note: SD: Standard Deviation
Table 3: Secondary outcomes of anti-glaucomatous molecules.
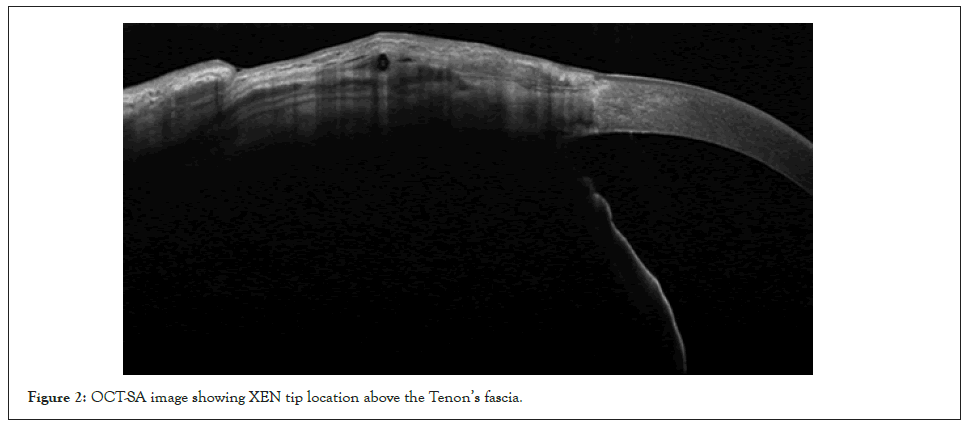
Figure 2: OCT-SA image showing XEN tip location above the Tenon’s fascia.
The XEN gel stent (Abbvie, Inc.) is a micro-invasive glaucoma device with a length of 6 mm and an inner lumen diameter either of 45 μm or 63 μm, which creates a filtration pathway from the anterior chamber to the subconjunctival space leading to an elevation of the conjunctiva, referred to as a filtering bleb. The proper functioning of the filtering bleb is mandatory for successfully surgical results. However, the normal process of the wound healing represents the first enemy after filtering glaucoma surgery, because fibroblast proliferation leads to bleb failure [7].
XEN gel stent is a hydrophilic tube composed of a porcine gel cross-linked with glutaraldehyde; its composition provides high biocompatibility with minimal tissue reaction, so as to minimize the postoperative fibrosis [8]. Then, the ab-interno technique provides for subconjunctival implant without conjunctival peritomy, with minimal tissue disruption and less surgical trauma. It is well known that reducing excessive intraoperative manipulation of conjunctiva and Tenon’s capsule prevents fibrosis [9].
Despite these measures, scar tissue often develops after surgery: previous studies report variable needling rates from 10% to 60% [10].
In this paper we have reported our modified technique of ab- interno implantation. The hypothesis is that the mechanical dissection performed with the subconjunctival injection of air and then BSS as first step of the surgery allows forcing the pre- existing adhesion between conjunctiva and Tenon’s capsule. It is well known that the conjunctiva of patients affected by glaucoma presents a chronic subclinical inflammation, in particular as a result of long-term topical treatments, thus leading to an increased risk of failure [11,12]. It is interesting to note that in our study the needling rate was only 4.34%.
We had also other advantages. The transformation of the virtual subconjunctival space in a real one permits an easier implantation of the device. In our study all implants were well positioned and straight, with the tip mobile, and in no case were excessively curled or required manipulation. Sometimes we performed an intraoperative needling, but we did not have significant subconjunctival bleeding, which can also lead to unwanted early scarring. Intraoperative bleeding, and the resulting blood clotting, induce the secretion of pro-inflammatory and pro-angiogenic cytokines, especially Vascular Endothelial Growth Factor (VEGF); that has a key role in inflammation and postoperative fibrosis [13,14]. We can assume that performing an intraoperative needling, after dissecting with air and BSS allows to interrupt the residual adhesion between conjunctiva and Tenon’s capsule but gently, and so to reduce the scarring process without bleeding. We had no case of intraoperative or post-operative hyphema. This could be one of the variables that lowered the needling rate to less than 5% in our cohort.
AS-OCT imaging technique provides high resolutions images in vivo and enables us to distinguish Tenon’s fascia from the conjunctiva; we can also determine the XEN tip location. Post- operative images confirmed the correct positioning of the tip, where visible, above the Tenon’s fascia as shown in Figure 2.
XEN gel stent has been designed to limit hypotony, lowering IOP safely in a predictable manner applying the Hagen-Poiseuille equation. This law postulates that the pressure differential across a tube with constant dimensions is proportional to the resistance to flow, and this is directly proportional to the length but inversely proportional to the radius of the tube to the fourth power. A tube with length of 6 mm and 45 μm of lumen size of diameter at average aqueous humor production of 2-3 μl/min provides a theoretical pressure drop of 8 mmHg, which prevents hypotony [15]. This isn’t always the case, however, in our cohort; we registered two cases (8.7%) of hypotony at post-operative day 1. None was high myopic (>6D), and one eye underwent a solo procedure, while the other a combined Phaco-XEN procedure. Thus, no risk factors have been identified [16]. However, both cases self-solved without the need of further interventions; none requires intracameral viscoelastic injection to manage a shallow anterior chamber. Previous studies report higher rates of hypotony, from 24.6% to 57%; the rates of choroidal detachment range from 1.4% to 19.8% [17-19]. There are different explanations such as direct toxicity to ciliary body by MMC; long-term use of topical anti-glaucomatous therapy (prostaglandins in particular) with permanent alteration of the uveoscleral outflow pathway, or an amount of aqueous flowing around the tube during the implant. However, in our cohort, we did not register any case of symptomatic or persistent hypotony, hypotony maculopathy and choroidal detachment.
We suggest a variation of the standard ab-interno technique, which is easy to perform, repeatable, more efficient and safer without an increase in costs. The dissection with air and BSS allows you to transform the subconjunctival space from a virtual to a real space, increasing the chances to correct positioning the device, reducing the intraoperative manipulation of the tissues, and thus improving the surgical outcomes. The limits of this study are the retrospective design, the follow-up period of 12 months and the small sample size.
Informed consent was obtained from all subjects involved in the study.
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Franco F, Serino F, Giansanti F (2023) Sparkling XEN Technique: An Innovation in the Surgical Implantation Technique of the XEN Gel Stent Device. J Clin Exp Ophthalmol. 14:945.
Received: 28-Feb-2023, Manuscript No. JCEO-23-21606; Editor assigned: 02-Mar-2023, Pre QC No. JCEO-23-21606 (PQ); Reviewed: 16-Mar-2023, QC No. JCEO-23-21606; Revised: 23-Mar-2023, Manuscript No. JCEO-23-21606 (R); Published: 31-Mar-2023 , DOI: 10.35248/2155-9570.23.14.945
Copyright: © 2023 Franco F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.