Forest Research: Open Access
Open Access
ISSN: 2168-9776
+44 1300 500008
ISSN: 2168-9776
+44 1300 500008
Research Article - (2014) Volume 3, Issue 2
Canopy structure and spatial distributions of understory vegetation are known to be closely correlated in forest community. The primary objective of this study is to examine the spatial relationships between the canopy heterogeneity and understory vegetation at multiple scales in an old-growth Douglas fir forest. A 12 ha plot located in the T.T. Munger Experimental Forest of Washington was stem-mapped, and the understory vegetation was surveyed along two 400 m transects by species and life form groups. Canopy structure of the plot was modeled utilizing a stem map and a crown geometry model. The three-dimensional canopy characteristics derived from the models were then used to assess the effects of canopy structure on understory vegetation at multiple resolutions using correlation and wavelet analysis techniques. Correlation results showed significant associations of understory species with the three-dimensional canopy structure. The majority of dominant herb species were highly associated with canopy opening, and they were more severely affected by lower canopy layers (heights of 10-35 m) than by dominant-codominant layers (heights of 40-50 m). The saplings of western hemlock had a significant negative association with lower canopy layers, while vine maples and the saplings of Pacific silver fir were positively correlated with dominant-codominant canopy layers. Wavelet analysis indicated that the relationship between canopy structure and understory vegetation was highly scale-dependent, i.e., understory variables responded differently to the heterogeneity of canopy patterns at different scales.
<Keywords: Forest canopy, Spatial heterogeneity, Wavelet analysis,Scale, Temperate forest.
Canopy structure is a key variable influencing many aspects of forest ecosystems [1-7]. Numerous studies have indicated that patterns of understory vegetation and physical environment (e.g., temperate, soil nutrients and moisture availability) were directly related to canopy openness [2,6-10]. For example, forest canopies provide microhabitats for plants, including mosses, herbs, and shrubs [11-13], and influence the composition of understory species, seedling regeneration, and microclimate conditions [2,6,7,10,14-17]. As the upper interface between forests and the atmosphere, forest canopies provide critical buffering from external disturbance for forest interior and create a microclimatic regime (e.g., sun flecks) below the canopy that drives understory processes. A high degree of structural diversity of the canopy provides high microsite variability, and consequently, diverse understory vegetation [2-4,13].
Studies have also demonstrated the importance of the three dimensional structure of forest canopies in determining microclimatic conditions in the understory [1,4,5,16,17]. Vertical structures, such as gap aspect ratio, tree height, and branch architecture, affect light regime and moisture level in the understory [9,10,18,19]. As a result, the differences in crown geometry and tree architecture can greatly influence the development of understory vegetation and successional dynamics [20,21]. Horizontal structural features lead to spatial variation in the understory microclimate and are important for developing and maintaining a structurally heterogeneous stand and diverse understory [2,8,13,21]. Radiation that reaches the understory is also affected by the spatial location of trees, stand age, density, and gap size [22-24]. Thus, understory conditions likely vary in a substantial manner due to different heights of canopy and horizontal locations because of the heterogeneity of the canopy structure. These height and location dependent variations in canopy structure are important for understanding the relationships between canopy and understory vegetation.
The primary objective of this study is to examine quantitatively the relationships between three-dimensional canopy structure and distribution of understory plants by investigating the variations in canopy structure at different heights and transect locations. We are particularly interested in examining whether the relationships between canopies at different heights and understory vegetation vary and if they are scale-dependent along transects.
Study area
The old-growth Douglas-fir (Pseudotsuga menziesii) forest at the Wind River Canopy Crane Research Facility (WRCCRF) is located within the T. T. Munger Research Natural Area of the Wind River Experimental Forest, 45o48’N, 121o58’W, on the Gifford Pinchot National Forest in southern Washington. The site is in the Wind River valley at 355 m elevation, deep within the southern Washington Cascade Range. A seasonally intermittent stream runs through the study site. The weather in the area is characterized as a temperate wet winter, dry summer climate with 2,528 mm of annual precipitation, with less than 10% occurring between June and September. Average annual snowfall is 2,330 mm. Mean annual temperature is 8.7oC. Soils are medial, mesic, Entic Vitrands that are deep (2-3 m), well drained, loams and silt loams, generally stone free, and derived from volcanic tephra [25].
This study area was located in a 500-year-old temperate forest stand dominated by Douglas fir and western hemlock (Tsuga heterophylla), with the tallest trees averaging 55 to 65 m (maximum 67 m). Other canopy species include western redcedar (Thuja plicata), western white pine (Pinus monticola), Pacific silver fir (Abies amabilis), grand fir (Abies grandis), and noble fir (Abies procera). Understory trees include Pacific yew (Taxus brevifolia) and Pacific dogwood (Cornus nuttallii). Dominant understory shrub species are vine maple (Acer circinatum), salal (Gaultheria shallon), and dwarf Oregon grape (Berberis nervosa).
Data collection and analysis
A 12ha (400 × 300 m) plot was established at the study site. Trees with diameters at breast height (DBH, 1.3 m above the ground) greater than 5 cm were measured and identified to species. Their cardinal coordinates (x, y) and the elevation (z) were measured using a computerized total surveying station (WILD TC600 Total Station, Leica Geosystems AG, Heerbrugg, St. Gallen, Switzerland).
Two 400 m line transects with east-west orientation were established in the plot (Figure 1). Understory plants and their life forms (i.e., shrubs, herbs, seedlings, and saplings) were sampled systematically using 2 2 m quadrates every 5 m along these two 400 m transects during the summer of 1996. Percent cover was recorded for each species and life form groups. For the shrubs and saplings, the coverage of different height classes (0-1 and 2-7 m) was also recorded.
Figure 1: Location and layout of two 400 m transects within a stem-mapped 12 ha (400 x 300 m) old-growth Douglas-fir forest in the Wind River Canopy Crane Research Facility in southern Washington. The species codes are the same as in Table 1. The sizes of circles represent the differences in tree size.
Canopy structure was modeled utilizing a stem map and a crown geometry model, and was tested with field-collected data [26]. The outputs of the model include the total projection of canopies for all trees in the plot, and canopy crosscuts at different heights (5-50 m with an interval of 5 m) for all species. Canopy openings along the two transects were also calculated using circular quadrates (radius = 5 m) located at the centers of the 2 × 2 m understory-sampling quadrates.
Frequency and average percent cover of all taxa present along the transects were calculated and tabulated. To assess species dominance, two additional diversity measures were calculated for each species: Shannon-Weiner Diversity Index (H-diversity) and evenness (Table 1). Abundance of each species was also plotted against distance along the transects to help assess distributional patterns and possible spatial relationships with canopy structure. Pearson correlation coefficients were used to examine the association between canopy structure (both horizontal and vertical) and understory vegetation.
To evaluate and compare the patterns of canopy structure and understory vegetation at multiple scales along the transects, we used wavelet analysis for each transect. Wavelet analyses, including wavelet transform and wavelet variance have been applied in several studies of ecological patterns at a range of temporal and spatial scales [24,27-29]. The advantages of using wavelet analysis include: 1) the ability to detect multi-scale patterns, 2) retain location information along the transect, and 3) no need to assume stationary in the data (unlike semi-variogram analysis, [27,29,30]. The wavelet transform is defined as
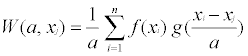 (1)
(1)
Where f(xi) is the data pattern function, g((xi-xj)/a) is a window function (i.e., wavelet) for a given range of scale a, centered at location xj along a transect, and n is the number of sample quadrates. We used a Mexican-hat wavelet to quantify peaks and troughs in the data. The resulting image was used to identify distribution patterns of canopy structure and understory vegetation at different scales along the transect and to determine whether they correspond with each other. In addition, we calculated the wavelet variance as:
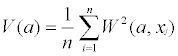 (2)
(2)
Wavelet variance indicates whether one scale contributes more or less than others to the overall pattern along a transect [27]. Pearson correlation coefficients were used to quantify the relationship between wavelet transforms of different variables at multiple scales. A program developed by Li and Loehle [31] was used to calculate W(a, x) and V(a).
Canopy structure and the composition of understory vegetation
The old-growth Douglas-fir forest was dominated by western hemlock (TSHE) and Douglas-fir (PSME) (Figure 1). Western hemlock was found in all canopy layers, with an average DBH of 30.8 cm, ranging from 4.8 to 132.1 cm, and height ranging from 4 to 57 m. Douglas-firs were generally taller than 45 m, with an average DBH of 106.2 cm. Most grand fir (),noble fir (), and red cedar (THPL) were in the dominant-co dominant layers (Figure 1), with averages DBH of 45.9 (firs) and 61.4 (cedars) cm. Pacific silver firs (ABAM) and Pacific yews (TABR) were found within the understory layer (Figure 1), with averages DBH of 11.1 and 14.3 cm, respectively. The canopy structure of this plot seemed very heterogeneous and complex, and appears as a mosaic of various patches (Figure 1). The average canopy opening was 19.6%.
Twenty-seven herbs and fourteen shrub species were recorded in a total of 162 plots along the two transects (Table 1). The abundancefrequency relationship for herb species was of negative-exponential shape (Figure 2a). Most herbaceous species occurred infrequently (< 15% of plots) and had low coverage (< 5%). No herb species with high frequency and abundance was found (Figure 2a). The three most frequent herb species were twinflower (Linnaea borealis), queen’s cup (Clintonia uniflora), and vanilla-leaf (Achlys triphylla), which appeared in more than 55% of the plots, although their cover ages were low (< 6%). There were some herb species, such as northern bedstraw (Galium boreale) and sword fern (Polystchum munitum), with very low frequency, but high abundance. Contrary to the herb species, the abundance-frequency relationship for shrub species was of positiveexponential shape (Figure 2b). There were several shrub species with high frequency and abundance, such as Salal and dwarf Oregon-grape, yet no shrub species with high frequency and low coverages.
| Code | Scientific Name | Common Name | (%) | Cover(%) | H-diversity | Evenness |
|---|---|---|---|---|---|---|
| Herbs | ||||||
| LIBO | Linnaea borealis | Twinflower | 62.3 | 2.21 | 3.78 | 0.82 |
| CLUN | Clintoniauniflora | Queen's cup | 56.8 | 1.83 | 4.17 | 0.92 |
| ACTR | Achlystriphylla | Vanilla-leaf | 56.2 | 5.99 | 3.51 | 0.78 |
| VISE | Viola sempervirens | Trailing yellow violet | 47.5 | 0.88 | 4.13 | 0.95 |
| TROV | Trillium ovatum | Western trillium | 45.1 | 0.66 | 4.15 | 0.97 |
| VAHE | Vancouveriahexandra | Inside-out flower | 29.6 | 2.61 | 3.09 | 0.80 |
| ANDE | Anemone deltoidea | Three-leaved anemone | 24.1 | 0.76 | 3.49 | 0.95 |
| PTAQ | Pteridium aquilinum | Bracken fern | 22.8 | 6.19 | 2.98 | 0.83 |
| XETE | Xerophyllum tenax | Bear-grass | 19.1 | 8.89 | 3.00 | 0.87 |
| CHUM | Chimaphila umbellata | Prince's-pine | 13.6 | 0.73 | 2.95 | 0.96 |
| GOOB | Goodyera oblongifolia | Rattlesnake-plantain | 11.7 | 0.84 | 2.59 | 0.88 |
| DIHO | Disporumhookeri | Hooker's fairybells | 11.1 | 1.81 | 2.41 | 0.84 |
| TITR | Tiarellatrifoliata | Three-leaved foamflower | 9.9 | 2.90 | 1.90 | 0.69 |
| CHME | Chimaphilamenziesii | Menzies 'pipsissewa | 6.2 | 0.60 | 2.21 | 0.96 |
| ADBI | Adenocaulon bicolor | Pathfinder | 4.3 | 5.21 | 1.67 | 0.86 |
| TRLA | Trientalislatifolia | Broad-leaved starflower | 3.7 | 0.83 | 1.64 | 0.92 |
| CASC | Campanula scouleri | Scouler's harebell | 2.5 | 1.25 | 1.31 | 0.95 |
| SMST | Smilacinastellata | Star-flowered false | 2.5 | 0.75 | 1.24 | 0.90 |
| POMU | Polystchummunitum | Sword fern | 1.9 | 8.83 | 0.71 | 0.65 |
| HIAL | Hieraciumalbiflorum | White flowered hawkweed | 1.9 | 1.17 | 1.00 | 0.91 |
| GABO | Galiumboreale | Northern bedstraw | 1.2 | 12.50 | 0.69 | 1.00 |
| BLSP | Blechnumspicant | Deer fern | 1.2 | 7.00 | 0.34 | 0.49 |
| GATR | Galiumtriflorum | Sweet-scented bedstraw | 1.2 | 3.25 | 0.67 | 0.96 |
| COCA | Cornuscanadensis | Dwarf dogwood | 1.2 | 0.50 | 0.69 | 1.00 |
| PTAN | Pterosporaandromedea | Pinedrops | 1.2 | 0.50 | 0.69 | 1.00 |
| VECU | Veronica cusickii | Cusick's speedwell | 1.2 | 0.50 | 0.69 | 1.00 |
| TRCE | Tricetumcernuum | Nodding trisetum | 0.6 | 2.50 | 0.00 | 0.00 |
| Shrubs | ||||||
| GASH | Gaultheria shallon | Salal | 87.0 | 18.54 | 4.41 | 0.89 |
| BENE | Berberis nervosa | Dwarf Oregon-grape | 72.2 | 20.65 | 4.36 | 0.92 |
| VAPA | Vacciniumparvifolium | Red huckleberry | 44.4 | 13.00 | 3.80 | 0.89 |
| ACCI | Acer circinatum | Vine maple | 32.1 | 17.38 | 3.30 | 0.84 |
| ROGY | Rosa gymnocarpa | Baldhip rose | 16.7 | 4.54 | 2.43 | 0.74 |
| VAAL | Vacciniumalaskaense | Alaskan blueberry | 8.6 | 9.89 | 2.16 | 0.82 |
| RUUR | Rubusursinus | Trailing blackberry | 5.6 | 1.22 | 2.03 | 0.92 |
| VAME | Vaccinum membranaceum | Black huckleberry | 3.7 | 1.92 | 1.63 | 0.91 |
| COCO | Coryluscornuta | Beaked hazelnut | 2.5 | 14.25 | 0.35 | 0.25 |
| SYAL | Symphoricarposalbus | Common snowberry | 2.5 | 1.25 | 1.09 | 0.79 |
| RHPU | Rhamnuspurshiana | Cascara | 1.2 | 0.75 | 0.64 | 0.92 |
| VAOV | Vaccinumovalifolium | Oval-leaved blueberry | 0.6 | 17.00 | 0.00 | 0.00 |
| HODI | Holodiscus discolor | Oceanspray | 0.6 | 8.50 | 0.00 | 0.00 |
| CONU | Cornusnuttallii | Pacific dogwood | 0.6 | 5.00 | 0.00 | 0.00 |
| ACCI (2 - 7 m) | Acer circinatum | Vine maple | 14.8 | 34.8 | 2.77 | 0.87 |
| Seedlings | ||||||
| TSHE | Tsugaheterophylla | Western hemlock | 82.7 | 2.1 | 3.86 | 0.79 |
| ABAM | Abies amabilis | Pacific silver fir | 26.5 | 0.7 | 3.52 | 0.94 |
| PSME | Psudotsuga menzeisii | Douglas-fir | 17.3 | 0.5 | 3.33 | 1.00 |
| TABR | Taxus brevifolia | Pacific yew | 5.6 | 0.5 | 2.20 | 1.00 |
| Saplings | ||||||
| TSHE | Tsugaheterophylla | Western hemlock | 40.1 | 27.2 | 3.64 | 0.87 |
| ABAM | Abies amabilis | Pacific silver fir | 35.8 | 16.0 | 3.39 | 0.83 |
| TABR | Taxusbrevifolia | Pacific yew | 3.1 | 8.9 | 1.43 | 0.89 |
| ABGR | Abiesgrandis | Grand fir | 1.2 | 8.8 | 0.60 | 0.86 |
Table 1: Frequency, average cover, H-diversityfor understory vegetation found along two transects in an old-growth Douglas-fir forest at Wind River Canopy Crane Area. The codes are the abbreviations of species scientific names.
The canopies were generally closed, but embedded with many openings (Figure 3). The average canopy opening along Transect 1 was 20.3%, with an 18.9% standard deviation, whereas the average opening for Transect 2 was 14.7%, with a 15.4% standard deviation. Transect 1 crossed several relatively large openings, and a trail crossed Transect 1 at around the 325 m location, while Transect 2 had continuous closed canopy for an extended distance (from 180 to 270 m) and relatively aggregated openings.
Figure 3: Distribution patterns along two 400 m transects for 1) model derived canopy opening (= [canopy opening area/(πr2) * 100], r = 5 m, %); 2) total herb cover (%); and 3) herb richness. The canopy openings were estimated from a canopy model, while herb total cover and richness were observed from 2×2 m quatrats along the transects.
Spatial associations between understory vegetation and canopy structure
There were obvious spatial associations between understory vegetation and the heterogeneity of canopy pattern along both transects (Figures 3 and 4). The covers (%) of vanilla-leaf, three-leaved anemone (Anemone deltoidea), queen’s cup, and twinflower were high when there were large canopy openings. Covers of these herbs were close to zero under closed canopies. The covers of the lower canopies (10-35m high) were negatively associated (P < 0.005) with covers of vanilla-leaf, queen’s cup, inside-out flower (Vancouveria hexandra), and three-leaved anemone, while vanilla-leaf, queen’s cup, inside-out flower, and three-leaved anemone were positively associated with each other (P < 0.001).
The spatial patterns of total abundance and total richness of all herbs were positively correlated with canopy openness (P < 0.001), and both were negatively correlated with the cover of lower canopies (10-35m high, P < 0.005). The covers and richness of herb species were larger for Transect 1 than those for Transect 2 (Figure 3a, 3b). Pathfinder (Adenocaulon bicolor), northern bedstraw, sweet-scented bedstraw (Galium triflorum), and nodding trisetum (Tricetum cernuum) only appeared on Transect 1, and only within the largest canopy opening on Transect 1. Hooker’s fairy bells (Disporum hookeri), broad-leaved starflower (Tiarella trifoliata), and inside-out flower were also associated with the largest canopy opening on Transect 1. Insideout flower had another peak at location 325 m of Transect 1, adjacent to a trail.
The total abundance of all shrubs was higher when there were large canopy openings around both transects (Figure 4a and 4b). Within a relatively large connected canopy opening across Transect 1 (Area 1-2 in Figure 4a), which was located at the edge of a trail, the covers of shrub species salal, Alaskan blueberry (Vaccinium alaskaense), and red huckleberry (Vaccinium parvifolium) reached their maximum. Salal had significant positive correlation (P < 0.05) with Alaskan blueberry, red huckleberry, and bear-grass (Xerophyllum tenax), while the latter three species tended to co-occur along the transects. Salal was negatively correlated (P < 0.05) with Douglas-fir canopies of all heights. Vine maples (2-7 m high) were positively correlated with the dominant-co dominant layers of the over story canopy (canopy heights at 40, 45, and 50 m, with P < 0.005, 0.005, and 0.001, respectively). The highest cover of vine maples occurred at the same locations where the dominant canopy cover reached a maximum.
The saplings of Pacific silver fir were also positively correlated with dominant-co dominant layers of over story canopies (canopy heights at 45 and 50 m, with P < 0.01 and 0.001, respectively), while western hemlock saplings were negatively associated with lower canopies (canopy height at 10, 15, and 20 m, with P < 0.01, 0.01, and 0.001, respectively).
Spatial scales determined by wavelet analysis
A high percentage of canopy openings were found at two locations (about 135 and 185 m) along Transect 1, as indicated by wavelet transforms (Figure 5a). Richness and total cover of herbs on Transect 1 reached their maximum at similar locations and scale ranges, clearly corresponding with high values of canopy openings (Figure 5a-5c). The correspondence of patterns among the variables on Transect 2 was not as obvious as on Transect 1 (Figure 5d-5f). There was a higher correspondence between wavelet patterns of total herb richness (Figure 5e) and model derived canopy openings (Figure 5d) when the distance along the transect was shifted to the left (Figure 5e), suggesting a possible lag effect.
Figure 5: Wavelet transforms of model derived canopy openings (a, d), herbaceous species richness (b, e), and total cover of herbaceous species (c, f) along Transect 1 and Transect 2, respectively. Different colors and shadings in a wavelet transform image represent changes of a variable (e.g., percent cover) at different locations (m, X-axis) and different scales (m, Y-axis). Red colors represent higher values of the variable, whereas dark blue represents lower values.
Along Transect 1, the correlations between wavelet transforms of canopy openings and total cover and richness of herbs were highest at scales of 25-40 m (Figure 6a). Along Transect 2, the correlation between canopy openings and total herb cover reached its maximum near the 45 m scale (Figure 6b), while at a similar scale, there was no correlation between canopy openings and total herb richness (Figure 6b). However, when moving along the transect of total herb richness to both east-west directions (negative if to the left and positive if to the right) at 5 m intervals, we found that the correlation between the two variables was maximized when this lag was equal to -15 m, with significant increases starting at around the 45 m scale (Figure 6b).
Figure 6: a) and b) are correlations of wavelet transforms among different variables along Transects 1 and 2, respectively, at different scales. c) and d) are the variances of wavelet transforms of different variables along Transects 1 and 2, respectively, at different scales. X-axis represents the scale (m) in all four graphs.
There was one peak in the wavelet variance for canopy openings on Transect 1 at about40 m scale, indicating that the dominant patch size of canopy openings tended to be about 40 m on Transect 1 (Figure 6c). There were two peaks on Transect 2 at scales around 17 and 115 m (Figure 6d). Further examining the canopy opening distribution along Transect 2 (Figure 3b) suggested that these two peaks may reflect the patchiness of canopy openings at these two scales. The wavelet transforms (Figure 5d) also reflected this trend that the first dominant patchiness was at around the 17 m scale and then this patchiness dissolved into larger scale dominance at around the 115 m scale.
Along Transect 1, the wavelet variances of total cover and richness of herbs were similar, reaching their peaks at scales around 45 m (Figure 6c). This showed that patches of high richness and total cover of herbs occurred mainly at the 45 m scale on Transect 1. There were similarities among wavelet variances of canopy openings, the total herb cover, and herb richness on Transect 1. While there was not an obvious peak in the wavelet variance of total herb cover along Transect 2, the wavelet variance of herb richness peaked at around the 17 m scale. The result indicated that the high herb richness on Transect 2 occurred primarily at this scale, and it corresponded to the dominant patchiness of canopy openings.
One of the contributions of this study was a unique view of three dimensional canopy structure (See authors’ previous publications for details, [5,26]. example of horizontal structure shown in Figure 1, and examples of vertical structure shown in Figures 7 and 8, both horizontally and vertically, in quantitatively characterizing the influence of canopy structure on understory vegetation at various scales. There existed vertical heterogeneity at different canopy heights along both transects (Figures 7 and 8). The canopy cover can be very different at different heights, e.g., in some locations on the transects, there were high canopy cover at lower canopy layer but low canopy cover at dominant- co dominant layer (Figure 7a, figure 7b, Figure 8a); while there were opposite situations in other locations on the transects (Figure 7c). In some locations, there were high canopy covers at intermediate canopy layer yet low canopy covers at lower canopy layers and dominant-co dominant layer (Figure 8b), suggesting that although the effect of the dominant- co dominant layer is important, the influences of the lower and intermediate canopies should not be ignored. Lower canopies can also play a critical role in the growth and spatial distribution of understory vegetation [16,26].
Figure 7: Along Transect 1, the canopy projection (%) at different tree heights (from height at 5 – 50 m, with an interval of every 5 m). a and b showed the locations where there were relatively high canopy covers (%) at lower canopy layers yet close to zero canopy cover at dominant-codominant layer, c showed the situation opposite to a and b.
The similarity of wavelet variances among canopy openings, herb total cover, and richness on Transect 1 reflected their correspondence at some scales (≈40-45m, Figure 6c), while on Transect 2 there was only correspondence between canopy openings and herb richness at scale around 17 m. It was not clear why the overall correlation between wavelet transforms of canopy openings and herb richness was the highest when the distance (horizontal-axis) of herb richness was shifted 15 m to the left along Transect 2, yet no lag effect was found along Transect 1. The reason perhaps is that the sun orientation is more influential when patch sizes (of both canopy openings and herbs) are smaller, thus less overlap and less direct (no lag) correlation. It was also not clear why the correlations of wavelet transforms of both total herb cover and richness with canopy openings were higher at the 45 m scale (Figure 6a and Figure 6b). It may be related to the geographical location of the site and how light penetrates this old-growth Douglas fir forest in which the heights of the dominant-co dominant layer are around 40 - 45 m.
Reproduction and growth of understory species are greatly restricted by light availability [26,32] and microclimate [9,10,15], which are determined by canopy structure. As a result, the distribution of understory species depends on the structure of the canopy [24,33,34]. The results of this study showed that patterns in composition and diversity of understory species had close associations with horizontal and vertical canopy structures, although their responses to different layers of the canopy varied.
It was interesting that the herb and shrub species in this oldgrowth Douglas-fir forest had the opposite trends in terms of the relationship between abundance and frequency (negative-exponential shape for herbs and positive-exponential shape for shrubs). Although the positive relationship between abundance and frequency for the shrubs is quite expected, the negative relationship for the herbs is worth an explanation. The latter relationship likely results from the limited number of gap patches and light availability in these patches in the understory. In this old-growth forest, light limits the growth of understory and large gap openings are few. Whenever such gaps are available, they are quickly occupied by the light demanding species, such as those locating at the bottom right corner of Figure 2a.
It is well known that species diversity increases and composition changes near forest or road edges because these edges provide a unique environment for many understory species [35]. In this stand, because of the trail that crossed Transect 1, it seemed more disturbed than Transect 2 it. Adjacent to this trail there were relatively large connected canopy openings cutting through both the north and south sides of the transect. These openings created high heterogeneity not only in canopy structure but also in the understory vegetation. Some understory herb and shrub species responded to these openings, suggesting that these species are associated with disturbance. The total abundance and richness of all herbs along Transect 1 were larger than the ones along Transect 2 because there were more canopy openings along Transect 1 than Transect 2.
In summary, in this study, canopy structure was quantified horizontally and vertically (See authors’ previous publications for details, [5,26]. Also example of horizontal structure shown in Figure 1, and examples of vertical structure shown in Figures 7 and 8), allowing quantitative characterization of the influences of canopy on understory vegetation at various scales [1,16,24]. Most dominant herb species that were positively associated with canopy openings were more negatively affected by lower canopy layers than by the dominant- co dominant layers. The spatial distributions of herb species were mainly affected by canopy openings and disturbance. The abundance of total shrubs had a positive relationship to canopy openness along the two sampled transects, although the interpretation on the associations between canopy structure and individual shrub species were not immediately clear. Wavelet analysis is a useful tool in characterizing the pattern changes and in comparing variables of interest across scales and locations along transects. The relationship between canopy structure and understory vegetation was highly scale-dependent, i.e., understory variables responded differently to the heterogeneity of canopy patterns at different scales.
Figure 8: Along Transect 2, the canopy projection (%) at different tree heights (with the same heights and intervals as in Fig. 7). a showed the similar situation as in Fig. 7a and b, b showed where there was high canopy covers in the intermediate canopy layers (20 – 30 m height) yet close to zero canopy covers at both low (5 – 10 m height) and dominant-codominant canopy layer.
This research was supported by grants from USDA Forest Service, Agreement No. PNW94-0541 and a Challenge Fellowship at Michigan Technological University.