Journal of Thyroid Disorders & Therapy
Open Access
ISSN: 2167-7948
ISSN: 2167-7948
Research Article - (2017) Volume 6, Issue 4
Introduction: Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by a symmetrical inflammation of the synovium, resulting in tenderness and destruction of bone and cartilage in various joints, particularly the smaller joints of the hands and feet. Although the cause of RA is unknown, autoimmunity plays a pivotal role in its chronicity and progression. Autoimmune thyroid disease (ATD) in the form of Hashimoto's thyroiditis and Graves' disease are all organ-specific. The relationship between RA and the thyroid gland has been studied extensively, with several studies demonstrating the autoimmune nature of thyroid dysfunction in RA.
Aims and Objectives: To study prevalence of thyroid dysfunction in rheumatoid arthritis patients.
Material and Methods: The present study was conducted in Postgraduate Department of Medicine, Government Medical College Srinagar over a period of 18 months in patients attending OPD clinic. The study was of prospective nature, and analytical cross sectional study.
Results: The mean age of patients was 48.2 ± 12.1.ESR was elevated in 89 (23.1%) patients while as it was normal in 296 (76.9%) patients. CRP was positive in 199 (51.7%) patients while as it was negative in 186 (48.3%) patients. RF was more than 3 times elevated in 238 (61.8%) patients, <3 times raised in 131 (34%) patients and negative in only 16 (4.2%) patients. Anti CCP was more than 3 times elevated in 300 (77.9%) patients, <3 times raised in 25 (6.5%) patients and negative in 60 (15.6%) patients.Anti-TPO antibodies were negative in 302 (78.4%) patients with rheumatoid arthritis and was positive in 83 (21.5%) patients.
Conclusion: Thyroid dysfunction is prevalent in patients with rheumatoid arthritis with percentage of 41.8%, subclinical hypothyroidism is the most common thyroid dysfunction encountered (37.9%) followed by overt hypothyroidism (3.6%), hyperthyroidism is very rare seen only 0.3% patients. Regular screening of patients is recommended.
Keywords: Autoimmune disease; Synovium; Autoimmunity; Rheumatoid arthritis; Autoimmune thyroid disease; Autoantigens; Нyroiditis Hypothyroidism; Overt hypothyroidism
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by a symmetrical inflammation of the synovium, resulting in tenderness and destruction of bone and cartilage in various joints, particularly the smaller joints of the hands and feet. Although the cause of RA is unknown, autoimmunity plays a pivotal role in its chronicity and progression. RA affects approximately 1.0% of the general population, women more often than men, and the inflammatory burden of the disease results in functional disability [1]. Several contributors to RA pathogenesis have been identified in recent years: genetic factors (shared epitope on locus HLA-DRB1, but also PTPN22, STAT4, 6q23 and TRAF1/C5), cigarette smoking, autoantibodies (rheumatoid factor (RF), anti-cyclic citrullinated protein antibodies (ACPA)), infectious agents, as well as nutritional and hormonal factors [2]. Ultimately, an interplay between these endogenous and exogenous factors has been postulated to break immunological tolerance and trigger the immunological response that manifests itself as RA [3]. The immunological activation of RA leads to infiltration of the synovium by an orchestra of immune cells like T and B cells, macrophages, dendritic cells and fibroblast-like synoviocytes, contributing to the proliferation of cell tissue (i.e. pannus formation) and neovascularization [4]. These immunological processes perpetuate systemic inflammatory responses, leading to a chronic, disabling disease which results in the inability to work and an impaired quality of life [5].
It has been widely observed that disorders with an autoimmune pathogenesis occur with increased frequency in patients with a history of another autoimmune disease (AD). The number of documented cases of co-occurrence of different autoimmune diseases in a single patient have increased in recent years.
Autoimmune thyroid disease (ATD) in the form of Hashimoto’s thyroiditis and Graves’ disease are all organ-specific. Autoimmune hypothyroidism is considered the most common autoimmune disease and its prevalence has been estimated to be up to 2%, with a higher concentration in older women [6,7]. ATD is characterized by the presence of antibodies against thyroglobulin, thyroid peroxidase, or thyrotropin receptor autoantigens. The relationship between RA and the thyroid gland has been studied extensively, with several studies demonstrating the autoimmune nature of thyroid dysfunction in RA [8,9].
In a systematic review analyzing the results of 54 studies that investigated the coexistence of ADs within individuals and 9 studies that examined within-family associations, these data supported an increased prevalence of ATDs among patients with RA [10]. In a separate study investigating the frequency of rheumatic diseases in patients suffering from ATDs, various rheumatic diseases were detected in 62% of patients with ATDs, one of which was RA [11].
The combination of RA and autoimmune thyroiditis was already recognized half a century ago [12-14]. More recently, the coexistence of RA and hypothyroidism was reassessed, but data on this relationship remains sparse [15,16]. As hypothyroidism and RA are both relatively common autoimmune diseases associated with increased CVD morbidity and mortality [17-21]. Further research into the interaction between these two diseases is worthwhile, both from a cardiovascular and from an immunological point of view.
To study prevalence of thyroid dysfunction in rheumatoid arthritis patients.
The present study was conducted in Postgraduate Department of Medicine, Government Medical College Srinagar and Associated Hospitals, Jammu and Kashmir, India over a period of 18 months in patients attending OPD clinic. The study was of prospective nature, and analytical cross sectional study. Ethical clearance was taken from the institution. We included 385 patients into the study based on an anticipated prevalence of thyroid dysfunction among rheumatoid arthritis and an absolute error of 5% with 30% prevalence and 95% confidence level.
Sample size was calculated based on these parameters:
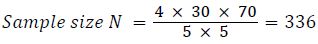
Considering a non-response rate of 15%.
Total sample size needed = 385
Inclusion criteria
The study included patients of rheumatoid arthritis who fulfilled European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) - 2010 criteria for rheumatoid arthritis and were screened for: triiodothyronine (T3), thyroxine (T4), free T4 (free thyroxine) thyroid stimulating hormone (TSH), anti-thyroid perioxidase antibodies (anti-TPO) antibodies.
Exclusion criteria
Patients with history of:
i)Surgical removal of thyroid gland.
ii)Any malignancy on radiotherapy and damage to thyroid.
iii)Patients on drugs causing hypothyroidism.
iv)Pregnancy
v)Patients on oral contraceptives
vi)Sepsis and serious underlying diseases
Patients attending Outpatient Department of Medicine at SMHS Hospital were evaluated for a history of thyroid disease, use of thyroid drugs or supplementation. Blood samples were obtained for the measurement of thyroxine, triiodothyronine, and thyroid stimulating hormone. Samples of blood were also obtained for the detection of rheumatoid factor and anti-TPO antibody. Taking all aseptic and antiseptic precautions about 3-5 ml of venous blood from median cubital vein was collected in clot activating vacutainer. The blood was collected after 10 to 12 hours of fasting. After clot formation, the samples were centrifuged at 4000 rpm to separate serum from the cells.
Serum aliquots were stored at 4°C to be run in batches. The samples were allowed to thaw prior to assay and mixed thoroughly. Hemolysed and lipemic samples were rejected.
Thyroid function test (TFT) T3, T4, FT3, FT4, TSH and anti-TPO were estimated by Chemiluminescent Microparticle Immunoassay (CMIA) method using ABBOTT ARCHITECT i1000 SR analyzer. It is a two-step immunoassay using Chemiluminescent Microparticle Immunoassay (CMIA) technology with flexible assay protocols, referred to as chemiflex. Patients with a serum level TSH of 0.35 mIU/ L-4.2 mIU/L was considered as normal. Levels more than or equal to 4.2 mIU/L with normal T4 and FT4 levels was considered as subclinical hypothyroidism and patients having raised TSH and low T4, FT4 levels were considered overt hypothyroid. T3 value of 0.6-1.6 ng/ml, T4 value of 4.5-11.7 ng/dl and FT4 levels of 0.8-1.7 were considered as normal.
Anti-TPO estimation was also done Chemiluminescent Microparticle Immunoassay (CMIA) method using ABBOTT ARCHITECT i1000 SR analyzer, for quantitative determination of IgG class of thyroid antibodies in human serum and plasma on the ARCHITEST iSYSTEM. A value of >5.6 ng/ml was taken as positive.
Statistical Analysis: The recorded data was compiled and entered in a spreadsheet (Microsoft Excel) and then exported to data editor of SPSS Version 20.0 (SPSS Inc., Chicago, Illinois, USA). Continuous variables were expressed as Mean ± SD and categorical variables were summarized as percentages. Frequency distribution tables, bar and pie charts were used for data presentation. Chi-square test or Fisher’s exact test, whichever appropriate, was used to determine association between various categorical variables. P-value less than 0.05 was considered statistically significant. All P-values were two tailed.
The mean age of patients was 48.2 ± 12.1. Most common age group was 35-49 years with 38.4% patients belonging to this group and next 30.1% belonging to 50-64 years age group. Out of a total of 385 patients, 334 (86.8%) were females and 51 (13.2%) were males.
ESR was elevated in 89 (23.1%) patients while as it was normal in 296 (76.9%) patients.
CRP was positive in 199 (51.7%) patients while as it was negative in 186 (48.3%) patients.
RF was more than 3 times elevated in 238 (61.8%) patients, ≤ 3 times raised in 131 (34%) patients and negative in only 16 (4.2%) patients.
Anti CCP was more than 3 times elevated in 300 (77.9%) patients, ≤ 3 times raised in 25 (6.5%) patients and negative in 60 (15.6%) patients.
Abnormalities of thyroid function test in the study population is illustrated in the Table 1.
| Thyroid Function Test | Number of Patients | Percentage | |
|---|---|---|---|
| TSH | <0.35 | 3 | 0.8 |
| 0.35-4.2 | 210 | 54.5 | |
| >4.2 | 172 | 44.7 | |
| T3 | <0.6 | 13 | 3.4 |
| 0.6-1.6 | 362 | 94.0 | |
| >1.6 | 10 | 2.6 | |
| T4 | <4.9 | 42 | 10.9 |
| 4.9-11.7 | 336 | 87.3 | |
| >11.7 | 7 | 1.8 | |
| FT4 | <0.8 | 21 | 5.5 |
| 0.8-1.7 | 326 | 84.7 | |
| >1.7 | 38 | 9.9 | |
Table 1: Thyroid function test among of the study patients (n=385).
Anti-TPO antibodies were negative in 302 (78.4%) patients with rheumatoid arthritis and was positive in 83 (21.5%) patients shown in Table 2.
| Anti-TPO | Number of Patients | Percentage |
|---|---|---|
| Negative | 302 | 78.4 |
| ≤ 3 times elevated | 2 | 0.5 |
| >3 times elevated | 81 | 21.0 |
| Total | 385 | 100 |
Table 2: Distribution of anti-TPO among study patients (n=385).
The most common thyroid dysfunction observed was subclinical hypothyroidism in 146 (37.9%) patients with elevated TSH and normal Free T4 levels. Overt hypothyroidism was seen in 14 (3.6%) patients and only a single patient (0.3%) was found to be hyperthyroid. Twenty (5.2%) patients were found to be euthyroid but had elevated anti-TPO antibodies. Total thyroid dysfunction was found in 41.8% patients as illustrated in Table 3.
| Parameter | Number of Patients | Percentage |
|---|---|---|
| Subclinical Hypothyroidism | 146 | 37.9 |
| Overt Hypothyroidism | 14 | 3.6 |
| Subclinical Hyperthyroidism | 1 | 0.3 |
| Euthyroid with elevated anti-TPO anti bodies | 20 | 5.2 |
Table 3: Spectrum of thyroid dysfunction in rheumatoid arthritis (n=385).
Although subclinical hypothyroidism was the most frequent abnormality observed in 37.9% patients, only 30% had concomitant anti-TPO raised and 71.4% patients of overt hypothyroidism had raised anti-TPO antibody.
Tables 4 and 5 shows RF was elevated in patients with overt hypothyroidism and in single patient with hyperthyroidism but it was not statistically significant. Similarly anti-CCP was more frequently elevated in patients with thyroid dysfunction. On the other hand, ESR and CRP were low in patients with thyroid abnormality. When the association between different inflammatory markers and thyroid function test was studied, it was observed that RF was significantly elevated in patients with subclinical hypothyroidism and the association was statistically significant with a p value of 0.007.
| RF | Total | P- value |
Anti CCP | Total | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | High | Normal | High | |||||||
| Subclinical Hypo-thyroidism | Yes | N | 1 | 145 | 146 | 0.007* | 25 | 121 | 146 | 0.513 |
| % | 0.7% | 99.3% | 100.0% | 17.1% | 82.9% | 100.0% | ||||
| No | N | 15 | 224 | 239 | 35 | 204 | 239 | |||
| % | 6.3% | 93.7% | 100.0% | 14.6% | 85.4% | 100.0% | ||||
| Total | N | 16 | 369 | 385 | 60 | 325 | 385 | |||
| % | 4.2% | 95.8% | 100.0% | 15.6% | 84.4% | 100.0% | ||||
| Overt Hypo-thyroidism | Yes | N | 0 | 14 | 14 | 1.000 | 2 | 12 | 14 | 1.000 |
| % | 0.0% | 100.0% | 100.0% | 14.3% | 85.7% | 100.0% | ||||
| No | N | 16 | 355 | 371 | 58 | 313 | 371 | |||
| % | 4.3% | 95.7% | 100.0% | 15.6% | 84.4% | 100.0% | ||||
| Total | N | 16 | 369 | 385 | 60 | 325 | 385 | |||
| % | 4.2% | 95.8% | 100.0% | 15.6% | 84.4% | 100.0% | ||||
| Hyper-thyroidism | Yes | N | 0 | 1 | 1 | 0.835 | 0 | 1 | 1 | 1.000 |
| % | 0.0% | 100.0% | 100.0% | 0.0% | 100.0% | 100.0% | ||||
| No | N | 16 | 368 | 384 | 60 | 324 | 384 | |||
| % | 4.2% | 95.8% | 100.0% | 15.6% | 84.4% | 100.0% | ||||
| Total | N | 16 | 369 | 385 | 60 | 325 | 385 | |||
| % | 4.2% | 95.8% | 100.0% | 15.6% | 84.4% | 100.0% | ||||
Table 4: Association between different variables (RF and Anti CCP) with the thyroid disturbance status in RA patients.
| CRP | Total | P-value | ESR | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | High | Normal | High | ||||||
| Subclinical Hypo-thyroidism | Yes | N | 79 | 67 | 146 | 0.075 | 118 | 28 | 146 |
| % | 54.1% | 45.9% | 100.0% | 80.8% | 19.2% | 100.0% | |||
| No | N | 107 | 132 | 239 | 178 | 61 | 239 | ||
| % | 44.8% | 55.2% | 100.0% | 74.5% | 25.5% | 100.0% | |||
| Total | N | 186 | 199 | 385 | 296 | 89 | 385 | ||
| % | 48.3% | 51.7% | 100.0% | 76.9% | 23.1% | 100.0% | |||
| Overt Hypo-thyroidism | Yes | N | 9 | 5 | 14 | 0.280 | 13 | 1 | 14 |
| % | 64.3% | 35.7% | 100.0% | 92.9% | 7.1% | 100.0% | |||
| No | N | 177 | 194 | 371 | 283 | 88 | 371 | ||
| % | 47.7% | 52.3% | 100.0% | 76.3% | 23.7% | 100.0% | |||
| Total | N | 186 | 199 | 385 | 296 | 89 | 385 | ||
| % | 48.3% | 51.7% | 100.0% | 76.9% | 23.1% | 100.0% | |||
| Hyper-thyroidism | Yes | N | 1 | 0 | 1 | 0.483 | 0 | 1 | 1 |
| % | 100.0% | 0.0% | 100.0% | 0.0% | 100.0% | 100.0% | |||
| No | N | 185 | 199 | 384 | 296 | 88 | 384 | ||
| % | 48.2% | 51.8% | 100.0% | 77.1% | 22.9% | 100.0% | |||
| Total | N | 186 | 199 | 385 | 296 | 89 | 385 | ||
| % | 48.3% | 51.7% | 100.0% | 76.9% | 23.1% | 100.0% | |||
Table 5: Association between different variables (CRP and ESR) with the thyroid disturbance status in RA patients.
The relationship between thyroid dysfunction and rheumatoid arthritis (RA) is a debatable subject and many studies have successfully demonstrated the autoimmune nature of thyroid dysfunction in RA [8,9].
The mean age of 48.2 ± 12.1 years in our study matches with that of Jorge Cardenas et al [22] and Malgorzata et al [23]. In many studies, it was also observed that ATTD was more frequently seen in females (Magnus [24], Scofeild [25]). In our study in the group of patients with subclinical hypothyroidism, 90.4% were females as also seen in study by Amany A. Mousa et al. [26].
We found that the inflammatory markers like ESR and CRP were not significantly raised in the studied population (23.1% and 51.7% respectively). These findings were also demonstrated by Mousa and others [26] who found high blood pressure and low CRP in hypothyroid patients.
The most common thyroid dysfunction observed was subclinical hypothyroidism seen in 37.9% of the patients which is very high (37.9%) when compared to general population in the same geographical area, Kashmir. (Hamid Bashir and others [27]--21.56%, Rama Jailkhani and others [28]--33%) The results of our study were similar to the study done by Rawdha Tekaya and others [29] in Tunisia who found that thyroid abnormalities were detected in 40% of the patients. In another study done by Ellatar and others [30], hypothyroidism was the most frequent abnormality detected in 24%, followed by subclinical hypothyroidism (4%) whereas hyperthyroidism was seen only in 1.3% of patients.
Clinically overt hypothyroidism was seen in 3.6% of the patients and only a single patient was hyperthyroid which was consistent with study done by Mosli et al. [31] These finding were also consistent with many earlier studies in which thyroid hormonal dysfunction and/or autoimmune thyroid disease were present in 6% to 33% patients with rheumatoid arthritis [32,33].
In our study subclinical hypothyroidism was the most frequent of the thyroid dysfunction in 37.9% patients which according to literature is present in 9.4 to 21% of patients with rheumatoid arthritis [34-37]. This state of subclinical hypothyroidism serves as a risk factor for development of overt hypothyroidism and is further favoured by age, gender and anti-TPO antibodies [38]. Clinical manifestation of the thyroid disease is often preceded by the presence of characteristic organ specific antibodies that might occur in serum even before the overt clinical manifestation [39]. This was also observed in our study as 20 (5.2%) patients with normal thyroid functions were found to have positive anti-TPO antibodies. This finding further strengthens the pyramid hypothesis which suggests that subclinical hypothyroidism develops in clinically manifested hypothyroidism in approximately one quarter of the cases. Autoimmune disease such as rheumatoid arthritis may accelerate the progression of subclinical disease into clinical disease (Porkodi et al. [40], Raterman et al. [41]).
Anti-TPO antibodies were seen in 71.4% of patients with clinically overt hypothyroidism while in patients with subclinical hypothyroidism 30.1% had positive anti-TPO antibodies. The current study thus demonstrates that 5.2% patients with normal thyroid functions and 30.1% patients with subclinical hypothyroidism had raised anti-TPO antibodies. The presence of anti-TPO in patients with normal thyroid functions, though not clinically significant, can be the marker that precedes and heralds the development of overt disease in near future [39,42].
This is a similar situation observed with other antibodies like RF and anti-CCP which can be seen in the serum of healthy subjects almost 0.1-13.8 years before the development of RA [43]. These findings make imperative not only for the screening of thyroid functions in RA patients but also of the presence of anti-TPO antibodies as the ATD marker in RA patients.
This trend of increased prevalence of anti-TPO antibodies is consistent with previous studies (Mousa et al.) [26]. It appears that there is association between the two antibodies i.e. AITD and RA. This association may be due to their common autoimmune etiology but the exact pathogenic mechanism is not known. Many theories have been proposed to explain this co-existence, one of which suggested that auto-reactive cells responsible for the underlying pathology in RA may provoke an autoimmune thyroditis. Environment and genetic factor may also precipitate these events [41]. Rolands and others [22] found prevalence of autoimmune thyroid disease was 9.8% while as the frequency of antibodies was 37.8% for anti-TPO in their study. The literature disclosed a geographical variation of autoimmune thyroid disease in rheumatoid arthritis ranging from 0.5% to 27%.
We also observed that there was a statistically significant association between RF-positive RA and subclinical hypothyroidism where in 99.3% of the patients with subclinical hypothyroidism had high RF (p=0.007). Anti-CCP was also raised in patients of subclinical hypothyroidism, clinically overt hypothyroidism and hyperthyroidism but this association was not statistically significant. It was interesting to note that CRP and ESR were not raised in patients with thyroid dysfunction. On the contrary, it was observed that 80.8% patients with subclinical hypothyroidism had normal RF and 92.9% patients with clinically overt hyperthyroidism had normal RF. The relationship between the presence of thyroid dysfunction and RA disease activity needs to be further observed. Besides, the response of the levels of inflammatory markers to treatment of the thyroid disease should also be studied.
Thyroid dysfunction is prevalent in patients with rheumatoid arthritis with percentage of 41.8%, subclinical hypothyroidism is the most common thyroid dysfunction encountered (37.9%) followed by overt hypothyroidism (3.6%), hyperthyroidism is very rare seen only 0.3% patients. The results of our study were similar to the study done by Rawdha Tekaya and others [29] in Tunisia who found that thyroid abnormalities were detected in 40% of the patients. In another study done by Ellatar and others [30], hypothyroidism was the most frequent abnormality detected in 24%, followed by subclinical hypothyroidism (4%) whereas hyperthyroidism was seen only in 1.3% of patients.
Regular screening of patients is recommended. Prevalence of thyroid disorders in rheumatoid arthritis patients was found to be significantly related to rheumatoid factor status, but no significant correlation was seen in other variables like ESR, CRP, anti-CCP. The increased prevalence of anti-TPO in patients with normal thyroid function may also serve as a marker that precedes and heralds the development of overt disease in near future; hence we recommend screening for anti-TPO antibodies in this population.