Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2022)Volume 11, Issue 4
Sperm chromatin undergoes structural changes during spermatogenesis causing high condensation. This nuclear exchange is considered to be a predictor of sperm-fertilization capacity and pregnancy outcome. Various studies have shown a relationship between male infertility and sperm protamine deficiency, and it has been suggested that it may be an important determinant of sperm DNA integrity. Acidic aniline blue staining is often used with glutaraldehyde fixation and stains histones due to lysine’s in the sperm nucleus. Research has shown that the level of histones in sperm nuclei is closely related to DNA damage and infertility. Sperm chromatin condensation is expressed by the presence of protamine in sperm nucleus, that is, the absence of histone. In this study, we investigated how the aniline blue staining method can correctly determine sperm chromatin condensation when different fixation methods are applied. Four different fixatives were used in this study. The fixatives were applied with glutaraldehyde (2.5%), methanol/acetic acid Carney’s, (3/1), ethyl alcohol (98%), and Formaldehyde (10%) aniline blue dye as applied at conventional concentrations in cytological preparations. Of these fixatives, glutaraldehyde is widely used to display sperm histones. Students -T-test and repeated measures analysis of variance were performed to calculate differences in chromatin condensation (aniline blue negative) rates among different fixatives. When the chromatin condensation values obtained with glutaraldehyde fixation were compared with those obtained with methanol and alcohol fixation, statistically significant differences (p=0.014,p=0.0001) were observed. A positive correlation between Glutaraldehyde and Formaldehyde (p=0.51), and a negative correlation between Glutaraldehyde and Formaldehyde, methanol/ acetic acid and alcohol fixation (p=0.005,p=0.0005 Ve p=0.0005,p=0.007) were observed. The positive correlation of chromatin condensation with sperm concentration and motility was determined (p=0.012,p<0,001). According to the results obtained in this study, 2.5% glutaraldehyde and 10% Formaldehyde fixations were appropriate for determining chromatin condensation with aniline blue, but methanol/acetic acid and ethyl alcohol is not suitable for this purpose. It was observed that the alcohol-based fixatives we tested in our study did not specifically show sperm lysine’s and caused the entire nucleus to be stained with aniline blue.
Sperm chromatin condensation; Aniline blue staining; Different fixatives
Routine semen analysis is a basic test for male infertility. However, it is difficult to understand the function and capacity of sperm cells by semen analysis alone [1-3]. Sperm chromatin condensation in the spermatozoa is an important marker of fertilization capacity and embryo development [4]. While the sperm head region develops during spermatogenesis, the organization of deoxyribonucleic acid (DNA)-related proteins takes place after spermatogenesis [5]. In this process, the transformation proteins TP1 and TP2, and protamines are placed around the DNA [5,6]. The retention of histones in the sperm head is known as chromatin condensation defect [7,8]. The extent of chromatin condensation defect can be determined by staining histones with aniline blue dye [9]. Found a condensation defect in 70% IUI cases with adverse pregnancy outcomes [9]. Chromatin condensation, defined as predomination, has a protective effect on sperm DNA and prevents DNA fragmentations [10,11]. A significant difference and a large chromatin defect were observed between the sperm samples of healthy donors and patients with asthenozoospermia and oligoasthenozoospermia using acridine orange staining [12].
Evidence on chromatin integrity shows a negative relationship between sperm chromatin material's insufficiency and the fertility potential of spermatozoa [13]. Therefore, chromatin integrity can be considered as a reliable predictor of a couple's ability to conceive.
In a study by Tarquem, it was found that all the tested fixatives, except for alcohol-ether and methanol-acetone, yielded similar results when aniline blue dye was applied to the sperm samples [14]. The sperm reacted differently in smears. Further, dye specificity at alkaline pH was lost. In general, chromatin is not stained; however, certain sperm heads are completely or partially stained.
In this study, we aimed to evaluate and quantitatively measure sperm chromatin condensation in normospermic patients by applying aniline blue dye with different fixatives. This study aimed to investigate the accuracy of sperm chromatin condensation when aniline blue is used by using 2.5% glutaraldehyde, methanol/acetic acid (3/1), 4% formaldehyde, and 96% alcohol.
Data source
This prospective controlled study involved sperm samples of male patients who visited the Bezmialem University Faculty of Medicine IVF lab for fertility testing between 2016 and 2017. The ethical committee of Bezmialem University approved the study on the 14.10.2015- Approval number 2015, 71306642-050.01.04.
Semen analysis and fixation
Semen analysis: In this study, men who applied to the infertility clinic and had normal sperm quality according to which 2010 criteria were selected. Oligospermic, asthenospermic, and teratozoospermia cases were not included in the study. From the semen samples collected (after 3-5 days abstinence) from the 42 patients who visited the infertility clinic, five smear preparations were prepared. Sperm motility and concentration were examined in 20 μl semen samples. The semen volume and pH were noted. The semen parameters were based on WHO 2010 criteria [15]. For sperm motility, the percentage of progressively motile, in situ, and standing sperm were calculated in 15 μl samples fixed between slides and coverslips. First, sperm preparation was stained with Diff Quick stain, and sperm morphology was examined. Of the other four samples, 1st sample with 2.5% glutaraldehyde, 2nd sample with 10% formaldehyde, 3rd sample with 98% ethyl alcohol for 30 minutes and 4th sample with methanol/acetic acid (3/1) at +4°C, 5 minutes fixed.
Application of acidic aniline blue staining
To perform this staining, a fresh sperm smear of each sample was air-dried and then fixed in 2,5% buffered glutaraldehyde in 0.2 M phosphate buffer (pH=7.2) for 30 min at room temperature. Each smear was treated with 5% aqueous AB stain (Sigma Co., St Louis, MO, USA) (5 g powder in 100 ml distilled water) in 4% acetic acid (pH=3.5) for 5 min. At least 200 spermatozoa were counted in each slide by light microscopy. Unstained or pale blue-stained cells and dark blue cells were considered normal and abnormal spermatozoa, respectively. At least 200 sperm cells were evaluated in each slide, and the percentage of abnormal spermatozoa was reported. A similar staining protocol was applied with other fixatives.
Statistical methods
Patients’ data were evaluated using The Statistical Package for the Social Sciences (SPSS) software version 24.0 (SPSS Inc., Chicago, IL) statistics program. The mean and standard deviation values were calculated as descriptive statistics. To compare the four fixation methods in terms of chromatin condensation values, analysis of variance was applied, and p<0.05 was considered significant.
As a result of aniline blue test application, the percentage of negative cells obtained by glutaraldehyde fixation of the aniline-negative cells (control) was compared with the chromatin condensation rates obtained by formaldehyde, methanol, and ethyl alcohol fixation (Figures 1-5). Table 1 shows the descriptive data of 42 cases. Chromatin condensation values obtained by glutaraldehyde fixation showed similar differences in formaldehyde fixation (p>0.05) and statistically significant differences in methanol and alcohol fixation (p=0.014, p=0.0001) (Table 2) (Figures 1 and 2). A negative correlation was found between glutaraldehyde and methanol, and glutaraldehyde and alcohol. However, there was a positive correlation between glutaraldehyde and formaldehyde and between methanol and alcohol (Table 3). A negative correlation was found between formaldehyde and methanol and formaldehyde and alcohol.
| n=42 | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|
| Sperm concentration x 106 | 15.6 | 135 | 44.07 | 34.19 |
| Total sperm x 106 | 46 | 441 | 158.74 | 121.72 |
| Progressive motility (%) | 29 | 71 | 48.48 | 14.04 |
| Nonprogressive motility (%) | 4 | 17 | 7.69 | 3.08 |
| Immotile sperm (%) | 25 | 78 | 43.83 | 11.62 |
| Normal morphology (%) | 4 | 8 | 4.36 | 1.97 |
| Aniline positive glutaraldehyde (%) | 22 | 48 | 30.71 | 5.93 |
| Aniline negative glutaraldehyde (%) | 52 | 78 | 69.29 | 5.93 |
| Aniline positive methanol (%) | 85 | 100 | 98.33 | 2.92 |
| Aniline negative methanol (%) | 0 | 15 | 1.67 | 2.92 |
| Aniline positive formaldehyde (%) | 24 | 43 | 32.43 | 5.19 |
| Aniline negative formaldehyde (%) | 57 | 76 | 67.57 | 5.19 |
| Aniline positive alcohol (%) | 95 | 100 | 99.14 | 1.63 |
| Aniline negative alcohol (%) | 0 | 5 | 0.86 | 1.63 |
Table 1: The descriptives of the semen samples’ data used in the study.
| Sum of squares | df | Mean square | F | P | ||
|---|---|---|---|---|---|---|
| Aniline negative methanol | Between groups | 244.467 | 19 | 12.867 | 2.699 | 0.014 |
| Within groups | 104.867 | 22 | 4.767 | |||
| Total | 349.333 | 41 | ||||
| Aniline negative alcohol | Between groups | 979.836 | 19 | 51.57 | 9.116 | <0.001 |
| Within groups | 124.45 | 22 | 5.657 | |||
| Total | 1104.286 | 41 | ||||
| Aniline negative formaldehyde | Between groups | 69.11 | 19 | 3.637 | 1.999 | 0.06 |
| Within groups | 40.033 | 22 | 1.82 | |||
| Total | 109.143 | 41 | ||||
Note: P: significant;*p<0.05, significant p-values are written in bold
Table 2: Comparison of chromatin condensation values (percentage of aniline blue negative spermatozoa) in the table as anova test result with other fixation methods and glutaraldehyde fixation result.
| Aniline negative glutaraldehyde | Aniline negative methanol | Aniline negative formaldehyde | ||
|---|---|---|---|---|
| Aniline negative methanol | r | -0.483 | ||
| p | 0.001 | |||
| n | 42 | |||
| Aniline negative formaldehyde | r | 0.849 | -0.393 | |
| p | <0.001 | 0.01 | ||
| n | 42 | 42 | ||
| Aniline negative alcohol | r | -0.452 | 0.809 | -0.347 |
| p | 0.003 | <0.001 | 0.024 | |
| n | 42 | 42 | 42 | |
Note: Pearson Correlation coefficient; p significant; n: number of patients;P<0.05 is significant, significant p-values are written in bold.
Table 3: Correlation analysis of the fixative methods.
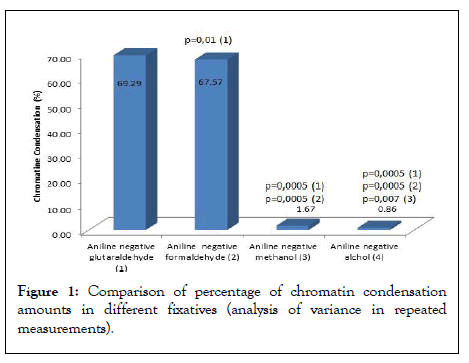
Figure 1: Comparison of percentage of chromatin condensation amounts in different fixatives (analysis of variance in repeated measurements).
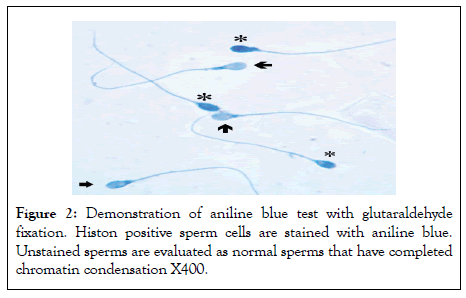
Figure 2: Demonstration of aniline blue test with glutaraldehyde fixation. Histon positive sperm cells are stained with aniline blue. Unstained sperms are evaluated as normal sperms that have completed chromatin condensation X400.
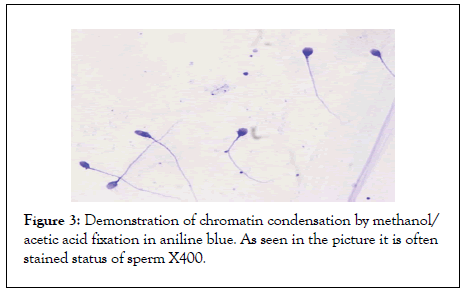
Figure 3: Demonstration of chromatin condensation by methanol/ acetic acid fixation in aniline blue. As seen in the picture it is often stained status of sperm X400.
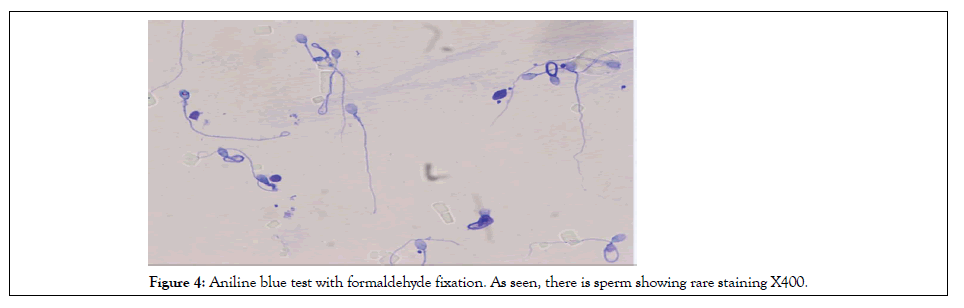
Figure 4: Aniline blue test with formaldehyde fixation. As seen, there is sperm showing rare staining X400.
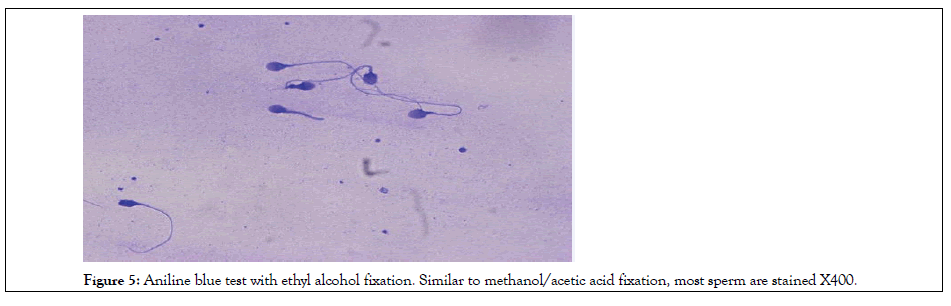
Figure 5: Aniline blue test with ethyl alcohol fixation. Similar to methanol/acetic acid fixation, most sperm are stained X400.
Table 4 shows the relationship of aniline-negative reaction with sperm parameters. The positive correlation of chromatin condensation with sperm concentration and motility was determined. The features of aniline blue staining are shown in Figures 2-5.
|
Sperm concentration | Progressive motility | Normal morphology | Aniline negative glutaraldehyde | |
|---|---|---|---|---|---|
Sperm concentration |
r | 1 | 0.375 | 0.211 | 0.386 |
| p | 0.014 | 0.179 | 0.012 | ||
| n | 43 | 42 | 42 | 42 | |
Progressive motility |
r | 0.375 | 1 | 0.586 | 0.536 |
| p | 0.014 | <0.001 | <0.001 | ||
| n | 42 | 42 | 42 | 42 | |
Normal morphology |
r | 0.211 | 0.586 | 1 | 0.044 |
| p | 0.179 | <0.001 | 0.782 | ||
| n | 42 | 42 | 42 | 42 | |
Note: r: Pearson Correlation coefficient, p significant, n: number of patients;*p <0.05 is significant (2-tailed), significant p-values are written in bold.
Table 4: Correlation analysis of sperm parameters with percentage of Aniline blue negative spermatozoa
Haploid male gamete cells typically package their DNA from the somatic cell nucleus to a volume of 10% or less. To achieve this extraordinary level of compression, spermatozoa replace most of the histones with smaller, highly basic arginine and cysteine-rich protamines. One reason for such a high compression is that it can help optimize the nuclear shape and support the ability of the sperm to swim in the female reproductive system [16]. Recent research has focused on the transfer of altered paternal histones to the zygote by spermatozoon and epigenetic reprogramming of the zygote after fertilization [10,17]. When sperm functions were examined in infertile men, it was observed that sperm chromatin abnormalities were more dominant [18,19]. Similar to the tests in which the integrity and quality of DNA are analyzed, tests that examine DNA packaging and maturation have also been developed [20,21]. During spermatogenesis, nuclear proteins are replaced by protamines, predominantly in somatic cells, and called histones and play an essential role in spermatozoa.
Disulfide-bridged protamines are essential proteins that fit the minor parts of DNA. They neutralize the negative charges of the phosphate groups, enabling the DNA to form a linear sequence to adapt to the major part of the neighboring chain. Generally, sperm DNA has 85% protamines, and approximately 15% of the histones localized to the nucleus [22]. This can be detected histochemically with the histone dye aniline blue [9,21]. Found that the mean chromatin condensation value (% aniline blue negative) in IUI cases was 26.75% in the successful pregnancy group and 16.75% in the failed pregnancy group. The researchers found that chromatin condensation has a positive correlation with sperm morphology and pregnancy rate. However, they did not find a correlation between sperm motility and sperm concentration in this study. However, in their later master’s thesis work (unpublished results), they achieved a low chromatin condensation in the oligozoospermic group. In the nucleus, and after the spermatozoon enters the oocyte, there can be a decondensation problem in the male genome. Therefore, the patients with abnormalities arising during sperm maturation and spermatogenesis are sub fertile or infertile [21]. Sperm chromatin abnormalities occur during or after the packaging of the DNA during spermatogenesis [23]. If damage by environmental factors, gene mutations, and chromosome abnormalities occurs during spermatogenesis, abnormalities occur in chromatin structure [24]. Chromatin condensation defects cause sperm DNA damage, and chromatin condensation measurement indirectly indicates the amount of DNA damage [25]. Although it is not known exactly how the DNA fragmentations in the human sperm originate, a few mechanisms have been proposed. Sperm chromatin packaging is vulnerable to programmed cell death and oxidative stress [12]. In previous studies, the rates of chromatin condensation anomalies and DNA fragmentation rates were similar [25]. Thus, techniques that can show excess histones remaining after maturation in sperm cells are fundamental. While most studies have not found a relationship between sperm chromatin condensation anomalies and sperm motility, morphology and concentration, a few studies found that condensation anomalies correlated with DNA fragmentation [12,26]. Researchers generally agree that protamine anomalies are associated with errors in spermatogenesis [21,24]. Found that sperm protamine deficiencies were associated with sperm concentration, motility, morphology, and DNA fragmentation [21]. It has been shown in many studies that the abnormalities of chromatin structure and DNA damage progress in parallel [23,27].
Semen analysis is the first test to evaluate sperm concentration, motility, morphology, and viability. However, additional tests should be performed even when semen analysis yields normal results. Sperm chromatin tests in assisted-reproduction techniques have become very popular in recent years. Studies have revealed the importance of sperm chromatin condensation tests such as the Sperm Chromatin Structure Assay (SCSA). Studying the structure of sperm chromatin can help identify the cause of infertility. Various laboratory evaluation methods and medical protocols play a valuable role in assisted reproduction techniques and the diagnosis and treatment of male infertility. Ensuring that laboratory tests are executed accurately is also very important.
Various histochemical methods can be used to evaluate sperm chromatin condensation. Aniline blue, Toluidine blue and Chromomycin A3 are some examples [12]. In Aniline Blue staining, basic proteins are loosely associated with DNA and cannot bind to the normally packed normal sperm chromatin. The transition of histones to basic protamines during spermatogenesis neutralizes the DNA load and reduces the amount of absorbent DNA-specific dyes [26].
Aniline blue is an acidic dye used in the laboratory to evaluate sperm chromatin integrity. Sperms with damaged DNA reveal residual histones and tend to bind aniline blue dye [25]. Aniline blue helps determine the difference between lysine-rich histones and arginine-rich proteins. This technique meets the need for a positive reaction for lysine, revealing the differences in the sperm's basic nucleus proteins. Excessive of histone proteins in the sperm cell means that the amounts of lysines are also high; therefore, the blue-colored reaction. However, since sperm cells rich in protamine contain arginine/cysteine proteins, blue staining is not observed in these cases [25].
There are many reasons why a fixation process is needed. For example, in the transmission electron microscopy technique, dehydration requires cross-sectioning of cells before staining, embedding and measuring. In another technique, it may require the removal of certain cell components for the analysis to work. Immunostaining using primary and secondary antibodies for subsequent fluorescence imaging may require disruption of the cell membrane to allow antibodies to reach their targets within the cell. This is usually accomplished by fixation.
Fixation methods can be divided into four main groups with their chemical structures; aldehydes, alcohols, oxidizing agents and metallic fixatives or by their influence; cross-linking, dehydration, heat effects or acid effects [27]. Many researchers evaluating fixation methods for specific cells and tissues have indicated that the choice of fixation method is often a personal choice rather than the conservation properties of fixation, or depends on the comfort of a particular method. However, there is no one-size-fits-all method, and some fixatives are more effective than others in protecting certain cell components. Methanol or ethanol is an alcohol which dehydrate cells instantly. Many lipids are removed from membranes, proteins precipitate. Small soluble metabolites leak and cells shrink. It is suitable for immunostaining as it keeps intact epitopes, for nucleic acid protocols such as ISH, FISH, caryotype, or tanscriptomics, but all structures in the cell are modified, collapsed. On other hand Formaldehyde or Glutaraldehyde is an aldehyde, which mildly oxidize reduced carbon or nitrogen. It creates bonds between proteins, lipids or between lipids and proteins. Cell structures are maintained, membranes remain intact (but permeable) but epitopes might be modified, nucleic acids are modified and a general, non specific can be produced [28]. We observed that the alcohol-based fixatives we tested in our study did not selectively reveal sperm lysins and caused the entire nucleus to be stained with aniline blue.
The fixatives used in histology vary depending on the purpose of the study. Different fixatives can show different features of a tissue or cell. Visualization of sperm histones was the primary purpose of our study, so alcohol-based fixatives were not beneficial. In our study, we used a quantitative procedure to test the efficacy of aniline blue with different fixatives in visualizing histones. Undoubtedly, such tests must provide accurate results. In our study, formaldehyde fixation, similar to glutaraldehyde fixation, showed advantages in terms of being an economical and common fixative in applying the method. Besides, in our study, Carnoy’s solution (methanol/acetic acid) and ethyl alcohol, which are widely used in cytochemical samples, failed in the aniline blue test application. As a result, aniline blue, which shows sperm chromatin maturity, can be safely used with formaldehyde and glutaraldehyde fixation.
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
Citation: Irez T, Durmus T (2022) Sperm Chromatin Condensation Level: Depending on Fixatives. Andrology.11:262.
Received: 03-Aug-2022, Manuscript No. ANO-22-17690; Editor assigned: 05-Aug-2022, Pre QC No. ANO-22-17690(PQ); Reviewed: 24-Aug-2022, QC No. ANO-22-17690; Revised: 29-Aug-2022, Manuscript No. ANO-22-17690(R); Published: 05-Sep-2022 , DOI: 10.35248/2167-0250.22.11.262
Copyright: © 2022 Irez T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NO