Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Research Article - (2014) Volume 5, Issue 1
Context: The increase in malignancy of young women in the recent decades, combined with a significant improvement in long term survival after gonadotoxic chemotherapy, have brought about a ubiquitous interest in preservation of fertility in these young patients. The present study examines the effects of Sphingosine-1-Phosphate (S1P) on primary human granulosa cell cultures in-vitro as a possible protecting factor against Doxorubicin (DOX) and Cyclophosphamide associated toxicity. Understanding cytotoxic effects and gonadotoxicity in human luteinized Granulosa Cells (GC) may contribute to our understanding and preventing follicle loss.
Study objective: To examine the possible protective effect of S1P on chemotherapy induced gonadotoxicity, in human luteinized Granulosa Cells (GCs).
Design: Human GC’s were donated by women undergoing follicular aspiration for in vitro fertilization (IVF), after informed consent and institutional approval by ethics committee (IRB, Helsinki). The GCs were separated from RBC’s by centrifugation on ficoll and plated on multiwell plates for Lactate Dehydrogenase (LDH) assay, and on 6 well plates for flow cytometry. Each experiment was conducted in triplicates and repeated at least three times.
Results: S1P significantly protected GCs against Doxorubicin (DOX) toxicity, but inconsistently against Cyclophosphamide.
Conclusion: S1P may minimize the gonadotoxic effect of chemotherapy on human luteinized granulosa cells.
Keywords: Sphingosine-1-Phosphate (S1P); Chemotherapy; Gonadotoxicity; Fertility preservation
In the last decades, the survival rates for many malignant diseases that affect young adults have increased to 80-90% [1,2]. Today’s 5-years survival rate for many young age cancers exceeds 80-90% and 10-years survival rate over 75% [3]. Thus, the advance in treatment results in improved childhood, adolescent and young adults cancer long-term survival [3]. Moreover, from 14% to almost 100% of the adult female cancer survivors may suffer premature ovarian failure (POF) - early menopause [4-7] while only 8-13% of pre-pubertal girls who were treated for malignant diseases will experience POF [5,8]. According to several studies, chemotherapy induced amenorrhea may occur in 30-76% of women depending on the treatment protocol and age [9]. Furthermore, irreversible chemotherapy-associated amenorrhea and POF appears in over 90% of women treated with high dose chemotherapy and total body radiation [9]. A few possible avenues have been put forward for fertility preservation: cryopreservation of embryos or unfertilized ova, ovarian tissue cryopreservation and administration of Gonadotropin Releasing Hormone agonists (GnRH-a) as co-treatment during chemotherapy. However, although dramatic increase in success rates has been reported following various preservation techniques [1,4,5] none of these methods can completely guarantee future fertility, and therefore it is recommended to consider all the available methods in order to maximize the chance of fertility preservation.
Since S1P may decrease the gonadotoxic effects of chemotherapy [9,10] and prevent ova destruction by doxorubicin, we have investigated its effect on human Granulosa Cells (GC). The GCs multiply rapidly during follicle maturation and as such may become a target to gonadotoxic medications. By protecting the GCs we may possibly protect the follicles and more importantly the oocytes and thus, may possibly prevent POF [9].
Cells
Granulosa cells were donated by women undergoing follicle aspiration by the transvaginal route for assisted reproductive technology at “Carmel” and “Bnay-Zion” hospitals’ IVF units. The cells were obtained after informed consent and the protocol was approved by the hospitals’ Helsinki committees.
Granulosa cells were retrieved from 233 women. The indication for IVF was male factor in 50%, mechanical infertility in 20% and the rest due to unexplained infertility or PCOS. Six women were diagnosed with endometriosis. Follicular fluid was collected and transported to the lab on ice and GC’s were separated from blood cells using the separation protocol reported by Bruce R. Carr laboratory, Dallas, TX [11]. Briefly, the cells were centrifuged at 800 g for 7 minutes, and the supernatant was removed, and the cells were resuspended in growth medium containing Dulbecco’s Modified Eagle Medium (DMEM) : Nutrient Mixture F-12 (Ham’s) (1:1) (DMEM/F12), 10% Fetal Bovine Serum (FBS), 1% insulin, human transferrin, selenous acid and linoleic acid (ITS+LA), 1% Penicillin-Streptomycin Amphotherycin B, 1.25% L-Glutamine (all from Biological Industries, Beit-Haemek, Israel), 0.01% Gentamycin (Teva, Petach Tikva, Israel). The cells were loaded on 5 mL ficoll premium 1.084 (GE Healthcare Bio-Science AB, Uppsala, Sweden) in 15 mL conical tubes. Granulosa cells from each patient were loaded in separate tubes in 2 mL medium; GCs aspirated from up to 20 follicles were loaded per tube. Cells were centrifuged for 15 minutes at 600 g to separate them from blood cells. GCs were separated, medium was added and the cells were resuspended and centrifuged again for 10 minutes at 800 g. Viability assessment was performed using trypan blue staining, and the cells were counted using hemocytometer.
The cells were seeded in multiwell plates according to experiments’ needs. 20,000 cells/well in 96 well plates in 200 μL medium or 250,000 cells/well in 6 well plates in 2 mL medium.
Experiments
Doxorubicin+S1P: Doxorubicin (DOX) (Pharmachemie BV, Haarlem, Holland or EBEWE Pharma, Unterach, Austria) was a gift from RAMBAM health care campus pharmacy as pure solution of 2 mg/mL and divided to 0.1 mL aliquots and stored at -20°C.
S1P (Bio-Lab LTD, Jerusalem, Israel) in powder was dissolved in methanol according to manufacturer’s instructions to a stock of 1 mg/mL and kept at -20°C. The experiments were conducted 2-7 days after seeding. DOX at a concentration of 2 mg/mL was diluted to the experiment concentration using low-protein medium containing DMEM/F12, 0.5% FBS, 1% ITS+LA, 1% Penicillin-Streptomycin Amphotericin B, 1.25% L-Glutamine and 0.01% Gentamycin.
S1P was diluted to the required concentration using low-protein medium containing DOX. 200 μL of medium were added to each well according to the experiment plan. The treated cells were incubated for 72 hours at 37°C in 5% CO2.
Cyclophosphamide+S1P: Cyclophosphamide [CPA] (Baxter Oncology, Halle/Westfalen, Germany) was a gift from RAMBAM health care campus pharmacy as pure solution of 20 mg/mL, divided to aliquots and stored at -20°C.
S1P in powder was dissolved in methanol according to manufacturer’s instructions to a stock of 1 mg/mL and kept in -20°C. The experiments conducted 2-7 days after seeding. CPA at concentration of 20 mg/mL was diluted to the experiment concentration using defined medium containing DMEM/F12, 0.1% Bovine Serum Albumin (BSA), 1% ITS+LA, 1% Penicillin-Streptomycin Amphotericin B, 1.25% L-Glutamine and 0.01% Gentamycin. Sample of S1P was diluted to the required concentration using the defined medium containing Cyclophosphamide. 200 μL of medium were added to each well according to the experiment plan. The treated cells were incubated for 72 hours at 37°C and 5% CO2.
Active metabolite of CPA+S1P: Phosphoramide Mustard (PM), the active metabolite of CPA, was a gift from Dr. Dror Meirow. The material was stored at -20°C and was protected from light. To each experiment, the material was weighed to get a stock solution of 1 mM in defined medium. Since the PM stability is unknown, for each experiment a fresh solution was prepared. The stock solution was diluted in defined medium to the concentrations needed to the experiment. Sample of S1P was diluted to the required concentration using the defined medium containing PM. 200 μL of medium were added to each well according to the experiment plan. The treated cells were incubated for 72 hours at 37°C and 5% CO2.
Lactate Dehydrogenase (LDH) activity Assay: LDH assay conducted according to manufacturer’s instruction (Roche Applied Science, Mannheim, Germany). Briefly, the assay was carried on in 96 well plates. GCs were seeded at a concentration of 20,000-25,000 cells/ well. Seeding at lower concentrations decreased the cells attachment resulting in high Standard Deviation (SD). Therefore, to reach the equivalent LDH concentration to those equivalents to 5000 cells/ well we diluted the collected medium 5 folds to a final volume of 100 μL. LDH solution was prepared shortly before use and kept in a tube covered with aluminum foil. 100 μL of LDH solution was added to each well. The plate was protected from light and agitated on a shaker for 30 minutes at room temperature. Results were read using ELISA plate reader (Zenyth 200, Biochrom, Cambridge, UK) at an optic density 492 nm and reference of 690 nm.
Flow cytometry: Cells were seeded on 6 well plates, 250,000 cells/well with 2 mL growth medium for 2-10 days. After 72 hours treatment the medium and cells were collected to 15 mL conic tubes. 200 μL trypsin were then added to each well and after 1 minute the cells removed using a scraper and the trypsin neutralized by adding 200 μL Fetal Bovine Serum [FBS]. The cells were then collected and added to the previously collected medium. Next, the well was washed with 1 mL Ca+2 and Mg+2 free PBS to collect the leftover cells after trypsinization. The cells were centrifuged at 470 g for 5 minutes, the medium was removed and replaced with 1 mL Ca+2 and Mg+2 free PBS, and the cells were centrifuged again at 470 g for 5 minutes. The supernatant was removed and replaced with 300-500 μL Ca+2 and Mg+2 free PBS. The cells were transferred to flow cytometry tubes (BD Falcon, Bedford, MA, USA), and counted using CyAn ADP flow cytometry (Beckman Coulter, Fullerton, CA, USA). Before counting, the cells were pipetted to disperse aggregates, and then 2 μL 7-amino Actinomycin D (7AAD) (Merck, Darmstadt, Germany) were added and the cells agitated by vortex for a few seconds before counting. The results were analyzed using Summit 4.3 software (Beckman Coulter, Fullerton, CA, USA).
Statistical analysis was performed using GraphPad Prism 5® software (GraphPad Software, Inc, San Diego, CA, USA). Gaussian, unpaired, two-tails t-test was performed, P<0.05 was considered significant.
Cell culture experiments
S1P effect on cell viability: At concentration 0.5-10 μM, S1P did not affect granulosa cell viability. However, at high concentrations of 50-100 μM, there was a small, increase in cell death.
S1P may protect GCs from DOX induced chemotoxicity: After determining that low S1P concentrations were not cytotoxic we examined the optimal DOX concentration for our experiments, the highest DOX concentration that S1P can protect against (Figure 1). The cells were exposed for 72 hrs. 1.5 μM DOX caused about 40% cell death and both S1P 1 μM and 5 μM protected the cells but only the higher S1P concentration could minimize toxicity for about 50% of the cells (reached C50). 1 μM DOX caused no significant cell death compared to the control, while 2 μM DOX was too gonadotoxic for S1P to protect against. We looked for the lowest S1P concentration that will result in 50% protection and accordingly decided to use S1P 1 and 5 μM, since lower concentrations were not protective (Data not shown).
Figure 1: S1P protection against DOX after 72 h incubation. The ability of S1P to protect the cells was a combination of two factors, DOX concentration and S1P concentration. Data normalized according to the following equation for calculating cytotoxicity (%) supplied by the LDH kit manufacturer .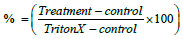 LDH assay was performed as described in the method section. Results are shown as mean ± SEM. N ≥ 32, *=P<0.05, ***=P< 0.001.
LDH assay was performed as described in the method section. Results are shown as mean ± SEM. N ≥ 32, *=P<0.05, ***=P< 0.001.
Can S1P protect GCs against CPA induced chemotherapy?: We examined whether S1P can also protect cells against CPA induced cytotoxicity. We used inactive CPA, which is commonly used in the oncologic clinical treatments. In-vivo, CPA is activated in the liver by cytochrome P450 (CYP) [12]. GCs also contain CYP aromatase, a member of the CYP superfamily enzymes. As might be expected our CPA experiments results were inconsistent; we preformed 40 experiments with different CPA doses and exposure times. In most cases, there were no significant differences between the control and the different concentrations of CPA, and after normalization of the results we received 30-40% cell death for both CPA 1 mg/ml and 2 mg/ml, and 20% with CPA 0.5 mg/ml. Paradoxically, in some experiments, CPA 0.5 mg/ml was even less cytotoxic than control (Figure 2A), while in others it was more cytotoxic than control (Figure 2B).
Figure 2a: The effect of cyclophosphamide on GCs after 72 h incubation. CPA induced significant cell death compared to control at all the tested concentrations. CPA 1 mg/ml and 2 mg/ml were significantly cytotoxic relative to control, while CPA 0.5 mg/ml was significantly less cytotoxic than control. At all CPA concentrations, S1P had no effect. LDH assay was performed as described in the method section. Results are normalization to Triton X-100,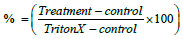 and shown as mean ± SEM. N≥42, **=P<0.01.
and shown as mean ± SEM. N≥42, **=P<0.01.
Figure 2b: S1P may protect GCs against CPA induced cytotoxicityrepresentative graph. S1P protected the GCs by decreasing cell death. CPA 0.5 mg/ml had, in this experiment, a small, statistically significant effect on the cells. On the other hand, CPA 2 mg/ml caused significant cell death and S1P abolished this effect completely. Right: The crude data shown as ΔOD, Left: The results normalized to Triton X-100. LDH assay was performed as described in the method section. Results shown as mean ± SEM. N=6, *=P< 0.05, ***=P< 0.001.
In-vivo, the CPA is activated in the liver to PM, therefore we assumed that the use of PM would result in higher cytotoxicity than the clinically used CPA. Therefore we also used PM, the CPA metabolite that is considered highly gonadotoxic, for the incubation with GC’s. We used both LDH (Figure 3A) and flow-cytometry (Figure 3B). At both techniques, we saw no consistent significant difference in the cytotoxic effect of CPA and PM.
Figure 3: PM cytotoxicity. Different concentrations of PM [Active CP] were given to the cells, and the results 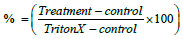 were examined by LDH assay (A) and flow-cytometry (B). PM [Active CPA] 200 μM was the only concentration that induced significant cell death, and S1P 5 μM showed significant protection (A). The effect of PM [Active CP] suggested a dose dependent pattern, but the different doses were not significant. Using the flow cytometry technique, we saw no significant effect on the cells and the dose response was more moderate than the one seen in the LDH assay. PM was not more effective than the inactive CPA. A: Crude data of LDH shown as ΔOD. B: flow cytometry analysis shown as percentages of dead cells out of total counted cells. Assays were performed as described in the method section. Results shown as Mean ± SEM (A) N ≥ 15 (B) N=3 repeats of 50,000 cells counted.
were examined by LDH assay (A) and flow-cytometry (B). PM [Active CPA] 200 μM was the only concentration that induced significant cell death, and S1P 5 μM showed significant protection (A). The effect of PM [Active CP] suggested a dose dependent pattern, but the different doses were not significant. Using the flow cytometry technique, we saw no significant effect on the cells and the dose response was more moderate than the one seen in the LDH assay. PM was not more effective than the inactive CPA. A: Crude data of LDH shown as ΔOD. B: flow cytometry analysis shown as percentages of dead cells out of total counted cells. Assays were performed as described in the method section. Results shown as Mean ± SEM (A) N ≥ 15 (B) N=3 repeats of 50,000 cells counted.
Can HGL5 granulosa cell line be used as a model?: GC cell line was uses in order to avoid the dependence on primary cells. The cell line was received as a gift from Dr. Bruce Carr’s lab at Dallas Texas, USA [11]. However, in several experiments, we saw no DOX effect and no S1P protection.
Although successful, none of the fertility preservation methods available for women is ideal, as none can ubiquitously guarantee 100% success. Therefore additional endeavors to minimize the gonadotoxic effects of chemotherapy are eagerly needed. Recently, Nakahara et al. [13] have found that treatment with S1P can inhibit the apoptosis of granulosa cells in response to oxidative stress induced by H2O2. They [13] have found that the protective effect of S1P was mediated by activating the PI3K/Akt pathway, and the antiapoptotic effect of S1P was mainly mediated through the S1P1 and S1P3 receptors. Whereas S1P, a known anti-apoptotic molecule, is present in human follicular fluid, as previously reported [13-15], we set to examine the possibility that S1P, may protect the ovarian granulosa cells from gonadotoxic chemotherapy, or attenuate the gonadotoxic effect of chemotherapy.
We aimed to find out if S1P, given exogenously, could protect the granulosa cells, somatic cells that surround the oocytes and are crucial for oocyte survival, and by doing so possibly protect fertility and ovarian function. We used both LDH assays and 7-AAD labeling for flowcytometry to measure cell cytotoxicity. While the LDH assay indicated that both 1 μM and 5 μM S1P may significantly protect the GCs against 1.5 μM DOX the flow-cytometry suggested that only 5 μM S1P is protective. Therefore, future extrapolation to in-vivo experiments, may suggest that a concentration of 5 μM S1P is recommended. When we examine the PM, although both the LDH assay and the flow-cytometry showed a dose dependent effect of the PM, there was a difference in the cell death and the cytotoxic effect measured. The LDH assay showed significant difference between PM concentrations and the control, while the flow cytometry showed no significant difference compared to control.
The difference in the S1P ability to protect GCs might be explained by the measuring techniques; while for the LDH assay we used an ELISA reader to measure the light absorption of dye created by enzymatic reaction, the flow-cytometry reads fluorescent illumination from single cells.
According to our results, S1P may protect primary granulosa cells from DOX, but may not protect GC cell line (HGL5).
We tried to extrapolate this protective effect using CPA, but with very limited success as CPA is a pre-drug and considered noncytotoxic, in-vitro, while its active metabolite, mainly PM is considered highly cytotoxic. Nevertheless, Raz et al. [16] found that CPA had a cytotoxic effect in vitro even in its inactivated state. Normally, CPA is activated in the liver by CPY [17]. GCs have a CPY aromatase enzyme, which is part of the CPY superfamily [18]. Our results indicate that CPA 1 mg/ml and 2 mg/ml caused 40-50% cell death which was higher than the control 30% cell death, but this difference in cytotoxicity has not been found to be statistically significant. This toxic effect may be induced by the native drug, or may indicate a possible activation to CPA active metabolite in the GCs. It is tempting to speculate that for in vitro exposure of cells containing CPY enzymes, CPA can be used as tissue specific treatment even if administered directly. However, this assumption needs further research.
Following our results that showed no statistical significance in cytotoxicity using CPA, a small amount of PM, an active CPA metabolite, was used on our cells, but did not yield better results than with the native CPA.
Both caused up to 40% cell death, not significantly different than the control. It is important to note that the PM used was not commercial, possibly explaining the low effect. Higher doses may possibly generate significant effects. However, we did observe that 5 μM S1P could significantly protect against the cytotoxic effect of 200 μM PM (Figure 2b).
Since both forms of CPA, active and not-active, showed no significant cytotoxicity compared to the control it is impossible to determine whether S1P can protect cells against CPA and its metabolites, in vitro. Our results suggest that there is a possibility that S1P can prevent CPA and PM induced cell death.
The ability of S1P to protect rodents’ and rhesus monkeys’ ovaries against chemotherapy and irradiation has been demonstrated before [10,19,20]. Therefore a method to use S1P for prevention of chemotherapy associated gonadotoxicity in human, without compromising the ability of chemotherapy to cure the malignant disease, is eagerly needed.
In conclusion, our study has shown a protective effect of S1P against Doxorubicin induced GC death, in vitro. However, no such consistent effect could be demonstrated, regarding cyclophosphamide, or its active metabolite, at the tested concentrations.
The advice and assistance of Dr Bruce Carr and his lab associates is sincerely appreciated, as well as the assistance and cooperation of Dr. Salim Hadad, director of the RAMBAM Health Care Campus pharmacy and his staff, and the assistance and cooperation of the laboratory and clinical staff of the IVF units at Carmel and Bnay-Zion medical centers. The supply of phosphoramide mustard by Dr. Dror Meirow’s lab is thankfully acknowledged.