Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Mini Review - (2023)Volume 12, Issue 4
Worldwide, approximately 190 million individuals are affected by infertility, of which 50% are male factor infertility cases. Basic microscopic examination constitutes the first-line evaluation of male fertility potential; however, because of normal results on basic semen analysis, approximately 15% of infertile males are diagnosed with idiopathic infertility. Both European Association of Urology (EAU) guideline and World Health Organization (WHO) laboratory manual have broadened the ambit of the platform of semen analysis with a dedicated section on Sperm DNA Fragmentation (SDF), which has benefits in not only identifying the cause of male infertility but also improving the outcomes of Assisted Reproductive Technologies (ART). Although SDF is a promising index of male reproduction, some gaps hinder the broader application of SDF in routine clinical practice. In this review, we discuss the limitations of SDF and present an update of the clinical utilization of SDF based on emerging knowledge, which enables a recalculation of the possibility of SDF incorporation in routine semen analysis to reshape the future of male infertility diagnosis.
Semen analysis; Infertility; Microscopic examination; Reproductive technologies
How to establish a standard pipeline for the evaluation of sperm DNA fragmentation?
Sample processing and preparation: DNA damage may occur during sperm processing and/or sample preparation. Furthermore, laboratory conditions, including prolonged in vitro incubation and cryopreservation, can induce a secondary negative effect on sperm DNA integrity. The sperm separation procedure, especially the involvement of centrifugation, can inadvertently impair SDF by inducing oxidation stress. Strategies to prevent these effect biases include a shorter incubation time, use of antioxidant enriched cryoprotectants and a centrifugationfree sperm separationmethod. Nevertheless, a fresh semen sample should be preferentially recommended for accurate interpretation of SDF [1-5].
Sperm DNA fragmentation assay
It is challenging to select a globally accepted golden assay from among the four WHO-recommended SDF detection methods because an ideal SDF diagnostic tool should confer multifaceted benefits (e.g. standardized protocol, consistent diagnostic performance, data accuracy, and data objectivity) [6]. Terminal Deoxynucleotidyl Transferase-Mediated Nick End-Labeling (TUNEL) labels the free 3’-OH-termini of DNA breaks with dUTP, and a Sperm Chromatin Structure Assay (SCSA) uses metachromatic acridine orange, with which intact double helixes and DNA fragments emit green and red fluorescence, respectively [7]. Both TUNEL and SCSA are coupled with flow cytometry for better testing reproducibility and reliability although flow cytometry has poor specificity in sperm identification. Therefore, the accuracy of the sperm DNA Fragmentation Index (DFI) is controversial because of nonsperm cell contaminations. Furthermore, the high demand of instrumentation and trained personnel limit TUNEL and SCSA utilization in clinical practice. Single-cell gelelectrophoresis assay (Comet) enables the quantification of DNA fragments via the separation of DNA fragmentswithin individual sperm nuclei during electrophoresis. Scoring software, such as CellProfiler, and CaspLab Biotool are used to improve the inter-observer reliability [8].
However, highintra-laboratory variability exists because of the lack of a standardized definition of sperm DNA break. Conventional Sperm Chromatin Dispersion (SCD) is a light microscopy-based method to evaluate the susceptibility of sperm DNA to acid denaturation, wherein a distinct halo represents the intact dispersed DNA loops and small or no halos indicate DNA damage. Despite the advantages of SCD, including minimum instrumentation requirements as compared to other methods, there are two frequent concerns: procedural cumbersomeness and subjective DFI interpretation. We recently investigated the advantages of SCD while aiming to overcome barriers to the usability of SCD. On the one hand, innovations in the in-gel denaturation technique have decreased the average SCD operation time by 40% than for conventional procedures [9]. On the other hand, the introduction of a novel automated semen analyzer dedicated to SDF evaluation significantly standardizes DFI interpretation. The LensHooke® X12 PRO semen analysis system (Bonraybio Co., Ltd, Taichung, Taiwan), which is trained by deep learning algorithms, can accurately identify the boundary between the sperm core and the halo for a standard interpretation of the halo-to-core ratio. Furthermore, X12 comprises an in-built autofocus optical lens and automatic XY table for multi-field examination that shortens the evaluation time to less than 5 minutes (Figure 1). In sum, technical innovations and artificial intelligence technology are enabling SDF standardization. Of note, the integration of the modified SCD assay and the automated semen analysis system would likely have the most potential for SDF standardization and the broadening of its usage in routine clinical practice [10]. Fragmentation evaluation: The LensHooke® X12 PRO semen analysis system uses deep learning algorithms to determine the levels of sperm DNA fragmentation.
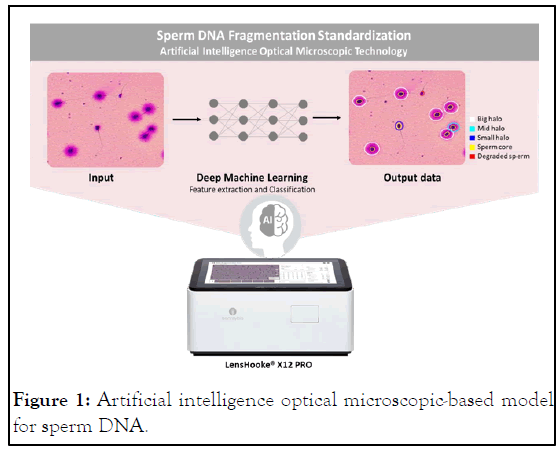
Figure 1: Artificial intelligence optical microscopic-based model for sperm DNA.
What are the benefits of including sperm DNA fragmentation in routine semen analysis?
Early diagnosis, precision medicine, and effective disease monitoring: In the current clinical scenario, following its categorization as an extended semen parameter, SDF evaluation is applied selectively to determine the cause of male infertility and/or ART failures. However, SDF tends to serve as a retrospective indicator for patients who have suffered failure(s) of natural conception and/or assisted reproduction attempts [11]. With the advent of SDF standardization and its routine use as first-line male infertility evaluation, we anticipate that SDFrelated influences can be minimized or prevented. Specifically, medical counseling and SDF-specific therapeutic strategies, such as antioxidant intervention, microscopic Testicular Sperm Extraction (TESE) and lifestyle modifications, can precede the ART cycle [12,13]. The combination of early diagnosis and early, precise treatment can improve the ART success rate and avoid the financial, social and emotional burdens of failed ART attempts. Furthermore, the standardized SDF analysis pipeline can provide objective andreliable data for the evaluation of medical intervention effectiveness and, alternatively, to monitor S DF-associated disease progression such as varicocele and leukocytospermia.
What limitations of sperm DNA fragmentation are yet to be addressed?
SDF evaluation can broaden the diagnostic ambit for male infertility and help predict pregnancy outcomes. However, only “global” SDF is available without the ability to differentiate between different types of DNA break: Single-Strand Break (SSB) and Double-Strand Break (DSB) that exert equivalent but exclusive impacts on reproductive outcomes. In particular, the role of DSB has garnered more attention [14]. Ribas-Maynou et al., performed Comet assay in neutral pH conditions (neutral Comet assay), which specifically enables the detection of DSB, and their results showed a significant DSB increase in recurrent pregnancy loss whereas the levels of global SDF using conventional SCD assay were unchanged. Similarly, delayed embryo development and impaired implantation rate were associated with significantly increased DSBs, but not global SDF. Therefore, the development of a standard algorithm for DSB measurement can help overcome the current limitations of SDF to provide a deeper insight into the etiologic workup and precision medicine in male reproduction [15].
For decades, SDF has been available as a male fertility test. However, the lack of a standardized analysis protocol has inevitably hindered SDF’s usefulness in clinical practice. With the technical innovations and advances in AI technology, SDF evaluation has been transformed into an easy-to-perform, timesaving, objective analysis that facilitates SDF availability for routine semen analysis as well as insights into preventive healthcare and precision medicine in male reproduction.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Wang TE, Hsu CT (2023) Standardization of Sperm DNA Fragmentation Evaluation: A Step toward Routine Semen Analysis. Andrology. 12:295.
Received: 29-Mar-2023, Manuscript No. ANO-23-22658; Editor assigned: 03-Apr-2023, Pre QC No. ANO-23-22658(PQ); Reviewed: 17-Apr-2023, QC No. ANO-23-22658; Revised: 02-May-2023, Manuscript No. ANO-23-22658(R); Published: 30-May-2023 , DOI: 10.35248/2167-0250.23.12.289
Copyright: © 2023 Wang TE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.