Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
+44 1300 500008
ISSN: 2167-0412
+44 1300 500008
Research Article - (2015) Volume 4, Issue 5
Ginger consists of the fresh or dried rhizomes of Zingiber officinale (Family: Zingiberacae). The dried rhizome powder of ginger is known as Sunth or Soonth. There are no standards available to determine the purity of the sunth powder which is sold in the local market. The objective of the present study was to evaluate Sunth powder (Dry ginger rhizome powder) and set standards to decide its purity. Pharmacognostic standardization was carried out to determine its microscopical characters, and also some of its quantitative standards. Microscopical studies were done by using the trinocular microscope. Total ash, water-soluble ash, acid-insoluble ash, alcohol and water-soluble extractive values were determined using the standard procedures. A preliminary phytochemical screening was also done to detect the different phytoconstituents present in sunth powder. Phytochemical analysis of the sunth powder was done and the presence of Gingerol was confirmed by using TLC techniques. These findings might be useful to supplement information with regard to its identification parameters, which are assumed significant in the way of acceptability of herbal drugs, in the present scenario, which lacks regulatory laws to control the quality of herbal drugs.
Keywords: Ginger, Zingiber officinale
TLC: Thin Layer Chromatography; LOD: Loss On Dryingq
Ginger is an important medicinal plant which majorly cultivated in countries like India, China, South East Asia, West Indies, Mexico and other parts of the world. Ginger consists of the fresh or dried rhizomes of Zingiber officinale (Family: Zingiberacae). The dried rhizome powder of ginger is known as Sunth or Soonth. The oil of ginger contains monoterpenes (phellandrene, camphene, cineole, citral, and borneol) and sesquiterpenes (zingiberene, zingiberol, zingiberenol, β-bisabolene, sesquiphellandrene, and others) [1]. It also contains several constituents such as gingerol, gingerdiol, and gingerdione, beta-carotene, capsaicin, caffeic acid and curcumin [2,3].
Ginger has a long history of traditional use. The British Herbal Compendium reported its action as carminative, anti-emetic, spasmolytic, peripheral circulatory stimulant and anti-inflammatory.
Several studies have demonstrated that ginger has beneficial effects to cancer prevention, pregnancy- related nausea and vomiting, chemotherapy nausea, nausea and vomiting after surgery and osteoarthritis. It has been shown that ginger acts as an inhibitor on cyclo-oxygenase (COX) and lipooxygenase, resulting in an inhibition of leukotriene and prostaglandin synthesis. Thus, ginger has been used as an anti-inflammatory because of its prostaglandin synthesis inhibition property. Ginger is therefore worthy of consideration as an analgesic in primary dysmenorrheal [4-12].
Medicinal plant materials are being adulterated in commerce due to many reasons such as similar morphological features, same name as written in classical text, presence of similar active principles in the substituted plant etc. that may badly affect the therapeutic activity of the finished products. Therefore, systematic identification is becoming essential in order to produce standardized finished herbal products.
The objective of the present study was to evaluate Sunth powder (Dry ginger rhizome powder) and set standard to decide its purity.
Materials
Sunth Powder (Mfg. By Anand Industries, Thane, India) was procured from local market. All the chemicals and reagents used in the present study were of analytical reagent grade.
Methods
Microscopic study of powdered plant material: Pinch of sunth powder was treated with chloral hydrate solution followed by staining with Iodine. The stained sample was placed on the grease-free microscopic slide along with the drop of glycerin and water (1:1) and then it was covered with a clean cover slip, observed under the trinocular microscope at 10X followed by 40X magnification. Photographs were taken using USB Microscope (2.0 M Pixels).
Preliminary Phytochemical evaluation of powder: The Sunth powder was evaluated for the presence of various phytoconstituents such as carbohydrates, proteins, alkaloids, glycosides, terpenes, steroids, flavanoids, tannins and saponins using commonly employed precipitation and coloration reactions reported in standard reference books [13-17].
Physiochemical analysis: Physiochemical parameters such as loss on drying (LOD), ash and extractive values were performed according to the official method prescribed and the WHO guidelines on quality control methods for medical plants material [18-20].
Thin layer chromatography (TLC): TLC of the sunth powder was performed as per the method reported in literature [21]. TLC of the methanolic extract was performed on precoated Silica gel 60, F254 plates (Stationary Phase) using mixture of ethyle acetate, formic acid and water in the ratio 88:6:6 as solvent system (Mobile Phase). 6-Gingerol was used as standard. Detection was done by dipping the plate in Anisaldehyde reagent followed by heating the plate at 100 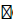 C in hot air oven. The Rf values for the separated spots were calculated and compared with RF value of pure 6-Gingerol and values reported in the literature.
C in hot air oven. The Rf values for the separated spots were calculated and compared with RF value of pure 6-Gingerol and values reported in the literature.
Microscopy
Microscopic examination of powder reported different types of starch grains such as spherical, ovoid, ellipsoidal or pear-shaped with varying size. Powder showed presence of long, thin-walled, non-lignified sclerenchymatous fibers, epidermis fragment and cork cells. Oil secretion cells (Parechyma) with suberized walls and containing a light yellowish or yellowish-brown, oily substance was also observed in the powder. Fairly large unlignified vessels with reticulate thickening were reported. The photomicrographs of different powder characters observed are depicted in Figures 1-6.
Figure 4: Parenchyma cells with yellow colored oil (10X).
Preliminary phytochemical screening
Preliminary phytochemical screening revealed the presence of carbohydrates, proteins, amino acids, terpenoids and steroids, flavonoids and tannins. The results of preliminary phytochemical results are summarized in Table 1.
| Sr.No. | Test | Observation |
|---|---|---|
| 1 | Carbohydrates (Molish test) | +ve |
| 2 | Proteins and Amino-acids | +ve |
| 3 | Alkaloids | -ve |
| 4 | Glycosides | -ve |
| 5 | Terpenoids and Steroids | +ve |
| 6 | Flavonoids | +ve |
| 7 | Tannins | +ve |
| 8 | Saponins | -ve |
Table 1: Preliminary phytochemical evaluation.
Physiochemical parameters
The results of ash values, extractive value and losses on drying are shown in Table 2.
| S. No. | Parameter | Result |
|---|---|---|
| 1 | Total Ash | 2.96 % |
| 2 | Acid insoluble ash | 0.88 % |
| 3 | Water soluble ash | 0.91 % |
| 4 | Water soluble Extractive | 11.7 % |
| 6 | Alcohol soluble extractive | 3.6 % |
| 7 | LOD | 2.2 % |
Table 2: The results of ash values, extractive value and losses on drying.
TLC
The fingerprint of the test solution was similar to that of the corresponding ginger reference sample reported in literature. Under white light the TLC plate of the test solution shows three violet zones at Rf ~ 0.26, Rf ~ 0.30, Rf ~033, Rf ~57 and Rf ~ 0.85. The Rf value of the spot appeared at Rf ~0.26 was corresponding to reference substance 6-gingerol. Other zones may be due to the presence of other phytoconstituents present in the ginger like 8-gingerol, 10-gingerol, Shogaol as reported in the literature.
Microscopical detection is easy, reliable and cost effective tool for detection of adulteration in medicinal plant materials. Present study has revealed an easy technique to identify sunth powder microscopically and this method can also be employed to detect the degree of adulteration in powdered raw medicinal plant materials as well. In conclusion, the present work was undertaken with a view to lay down standards which could be useful to detect the authenticity of sunth powder. Microscopic study and physiochemical standards can be useful to substantiate and authenticate the drug.
The author[s] declare[s] that there is no conflict of interests regarding the publication of this article.
Author is very much thankful to Dr. [Mr.] Paraag Gide, Principal of Hyderabad Sindh National Collegiate boards [HSNCB’s] Dr. L. H Hiranandani College of Pharmacy, Ulhasnagar for his continuous support and encouragement.