Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2014) Volume 5, Issue 2
N-Acryloyl-4-aminosalicylic acid (AAS) was synthesized and characterized using different spectroscopic techniques. The geometrical structures of the ligand are carried out by HF method with 3-21G basis set. The proton-ligand dissociation constants of AAS and its metal stability constants with (Mn2+, Co2+, Ni2+ and Cu2+) have been determined potentiometrically in monomeric and polymeric forms using 2,2’-azobisisobutyronitrile as initiator. The potentiometric studies were carried out in 0.1 M (KCl) and 20% (by volume) ethanol-water mixture. The effect of temperature was studied at (298, 308 and 318 K) and the corresponding thermodynamic parameters (ΔG, ΔH and ΔS) were derived and discussed. The dissociation process is non-spontaneous, endothermic and entropically unfavorable. The formation of the metal complexes has been found to be spontaneous, endothermic and entropically favorable.
Keywords: N-Acryloyl-4-aminosalicylic acid; Molecular structure; Potentiometry;Thermodynamics
Polymer complexes have been given a great deal of attention in recent years. The formation of chelates by polymers has widely been used for speciation [1], concentration, sepration [2] and recoveration [3] of metal ions. Stability constants are key parameters for the investigation of equilibria in solution and are very important in many fields such as industerial chemistry [4], enviromental studies [5], as well as medicinal [6] and analytical chemistry. Potentiometeric method is used to determine the average number of ligands coordinated with metal ion and, further, calculation the stability constants of polymermetal complexes [7-10].
Salicylic acid and its derivatives are also biologically important ligands, a well known and widely used derivative, aspirin, reduces the risk of many diseases associated with ageing and is used in the treatment of rheumatic fever, pain, the preventation of thrombosis in vascular system analgesic, antipyretic, anti-flammatory bowel diseases (IBD) and anti-tuberculosis drug [11,12].
As a part of our continuous work reporting on the determination of dissociation and stability constants of some organic compounds and their metal complexes by potentiometric techniques [13-16], we report here the proton-ligand dissociation constants of N-Acryloyl-4- aminosalicylic acid (AAS) and its metal stability constants with (Mn2+, Co2+, Ni2+ and Cu2+) in monomeric and polymeric forms. Furthermore, the corresponding thermodynamic functions are evaluated and discussed. The geometrical structures of the ligand are carried out by HF method with 3-21G basis set.
Materials
All the compounds and solvents used were purchased from Aldrich or Sigma and used as received without further purification. Acryloyl chloride was used without further purification. It was stored below -18 oC in a tightly glass-stoppered flask. 2,2’-Azobisisobutyronitrile (AIBN) was used as initiator for all polymerizations. It was purified by dissolving it in hot ethanol and left to cool. The pure material was being collected by filtration and then dried [17].
Preparation of N-Acryloyl-4-aminosalicylic acid monomer
N-Acryloyl-4-aminosalicylic acid monomer was prepared previously [13] by the reaction of equimolar amounts of acryloyl chloride and 4-aminosalicylic acid in dry benzene until the evolution of hydrogen chloride ceased forming a gray powder of AAS monomer (Figure 1).
Potentiometeric studies
N-Acryloyl-4-aminosalicylic acid solution (0.001 M) was prepared by dissolving an accurate weight of the solid in ethanol (AnalaR). Metal ion solutions (0.0001 M) were prepared from AnalaR metal chlorides in bidistilled water and standardized with EDTA [18]. Solutions of 0.001 M (HCl) and 1 M (KCl) were also prepared in bidistilled water. A carbonate-free sodium hydroxide solution in a 20 % (by volume) ethanol-water mixture was used as titrant and standardized against oxalic acid (AnalaR).
The apparatus, general conditions and methods of calculation were the same as in previous work [13-16]. The following mixtures (i)-(iii) were prepared and titrated potentiometrically at 298 K against standard 0.002 M (NaOH) in a 20 % (by volume) ethanol-water mixture:
i) 5 cm3 0.001 M (HCl)+5 cm3 1 M (KCl)+10 cm3 ethanol.
ii) 5 cm3 0.001 M (HCl)+5 cm3 1 M (KCl)+5 cm3 0.00l M ligand + 5 cm3 ethanol.
iii) 5 cm3 0.001 M (HCl)+5 cm3 l M (KCl)+5 cm3 0.001 M ligand + 10 cm3 0.0001 M metal chloride + 5 cm3 ethanol.
For each mixture, the volume was made up to 50 cm3 with bidistilled water before the titration. The titrations also were carried out in the presence of 5 ml of AIBN (0.001 M) as initiator for the polymerization step (Figure 1). These titrations were repeated for temperatures of 308 and 318 K. All titrations have been carried out between pH 3.0 and 11.0.
Measurments
Spectroscopic data were obtained using the following instruments: FT-IR spectra (KBr discs, 4000-400 cm-1) by Jasco-4100 spectrophotometer; the 1H NMR spectrum by Bruker WP 300 MHz using DMSO-d6 as a solvent containing TMS as the internal standard. The pH measurements were carried out using VWR Scientific Instruments Model 8000 pH-meter accurate to ± 0.01 units. The pH- meter readings in the non–aqueous medium were corrected [19]. The electrode system was calibrated according to the method of Irving et al. [20]. Titrations were performed in a double walled glass cell in an inert atmosphere (nitrogen) at ionic strength of 0.1 M KCl. Potentiometric measurements were carried out at different temperature. The temperature was controlled to within ± 0.05 K by circulating thermostated water (Neslab 2 RTE 220) through the outer jacket of the vessel.
The molecular structures of the investigated compound were optimized by HF method with 3-21G basis set. The molecules were built with the Perkin Elmer ChemBio Draw and optimized using Perkin Elmer ChemBio 3D software [21,22]. Quantum chemical parameters such as the highest occupied molecular orbital energy (EHOMO), the lowest unoccupied molecular orbital energy (ELUMO) and HOMO– LUMO energy gap (ΔE) for the investigated molecules are calculated.
IR spectra of N-Acryloyl-4-aminosalicylic acid
Examination of IR spectrum of the N-Acryloyl-4-aminosalicylic acid (AAS) shows a broad band appears at ~3458 cm-1 corresponding to amide νNH stretching vibration followed by medium broad band appears at 3006 and 2889 cm-1 which assigned to OH of phenolic, carboxylic OH, respectively [17]. This broadening is an evidence for the presence of weak hydrogen bond between OH and carboxylic groups [23]. IR spectrum of AAS exhibits a bands at 1879 and 1612 cm-1 is assigned to the antisymmetric streatching vibration of carboxylic and amidic carbonyl group, respectively. The bands at 1616, 1569 and 1415 cm-1 are assigned to the (C-H), ν(C=C) and ν(C-C) bands, respectively [24]. The bands in the region 1236-1106 cm-1 are due to the C-H in plane deformation, while the out-of-plane deformation vibration between 727-962 cm-1 and the C-C out-of-plan deformation at 500 cm-1 are assigned.
1H-NMR spectra
The 1H NMR spectra of AAS revealed six resonances at 10.80, 7.57, 7.28, 3.20, 2.53 and 2.10 ppm relative to TMS which may be assigned to an acid group COOH, CONH, aromatic protons, OH phenolic, vinylic –CH– and –CH2 protons, respectively [25]. The peak at (10.80/3.20/7.57) ppm, which is due to the exchangeable hydrogen-bonded carboxyl/hydroxyl/amine (COOH/OH/NH) proton, respectively which disappears upon exchange with D2O and can be associated with the COOH/OH/NH protons involved in intramolecular hydrogen bonding. The 1H NMR spectrum of the ASS monomer showed the expected peaks and pattern of the vinyl group (CH2=CH) δ 6.82 ppm (dd, J = 17, 11 Hz) for the vinyl CH proton and proton δ 6.32 ppm (AM part of AMX system dd, J = 17,1 Hz) for the vinyl CH2 protons, respectively. These peaks disappeared on polymerization while a triplet at δ 2.53 ppm (t, J = 7 Hz) and a doublet at 2.1 0 ppm (d, J = 7 Hz) appeared, indicating that the polymerization of AAS monomer occurs on the vinyl group [26]. It is worth noting that the rest of the proton spectrum of the monomer and polymer remain almost without change.
Molecular structure
The molecular structures of the N-Acryloyl-4-aminosalicylic acid (AAS) were optimized by HF method with 3-21G basis set. The molecules were built with the Perkin Elmer ChemBioDraw and optimized using Perkin Elmer ChemBio3D software. The calculated molecular structures for AAS are shown in Figure 2. Selected geometric parameters bond lengths and bond angles of AAS are tabulated in Table 1 and Figure 3.
| Theoretical | |||
|---|---|---|---|
| Bond lengths(Å) | |||
| O(9)-H(24) | 0.971 | N(12)-C(13) | 1.365 |
| C(6)-H(23) | 1.098 | O(10)-H(11) | 1.0 |
| C(3)-H(22) | 1.102 | C(5)-O(10) | 1.379 |
| C(2)-H(21) | 1.104 | C(7)-O(9) | 1.349 |
| O(8)-H(11) | 1.007 | C(7)-O(8) | 1.229 |
| N(12)-C(1) | 1.35 | C(4)-C(7) | 1.366 |
| C(16)-H(20) | 1.1 | C(6)-C(1) | 1.346 |
| C(16)-H(19) | 1.102 | C(5)-C(6) | 1.345 |
| C(15)-H(18) | 1.105 | C(4)-C(5) | 1.35 |
| N(12)-H(17) | 1.01 | C(3)-C(4) | 1.339 |
| C(15)-C(16) | 1.342 | C(2)-C(3) | 1.342 |
| C(13)-C(15) | 1.36 | C(1)-C(2) | 1.348 |
| C(13)-O(14) | 1.206 | ||
| Bond angles (°) | |||
| H(20)-C(16)-C(15) | 122.966 | O(9)-C(7)-O(8) | 119.333 |
| C(15)-C(13)-O(14) | 124.552 | O(9)-C(7)-C(4) | 125.455 |
| C(15)-C(13)-N(12) | 111.542 | O(8)-C(7)-C(4) | 115.212 |
| O(14)-C(13)-N(12) | 123.905 | C(7)-C(4)-C(5) | 114.09 |
| C(1)-N(12)-H(17) | 109.793 | C(7)-C(4)-C(3) | 124.218 |
| C(1)-N(12)-C(13) | 133.388 | N(12)-C(1)-C(6) | 126.721 |
| H(17)-N(12)-C(13) | 116.819 | N(12)-C(1)-C(2) | 115.026 |
| Torsional angles (°) | |||
| C(5)-C(4)-C(7)-O(9) | 178.807 | ||
| C(3)-C(4)-C(7)-O(8) | 179.382 | ||
| Intermolecular H- bond (Dimer) | |||
| Bond lengths(Å) | Bond angles (°) | ||
| O(9)-H(10) | 1.010 | O(26)-H(10)-O(9) | 176.73 |
| H(10)-O(26) | 1.075 | O(8)-H(28)-O(27) | 179.427 |
| O(27)-H(28) | 0.991 | O(11)-H(12)-O(8) | 164.782 |
| H(28)-O(8) | 1.072 | O(29)-H(39)-O(26) | 164.113 |
| O(11)-H(12) | 0.995 | ||
| H(12)-O(8) | 1.061 | ||
| O(29)-H(39) | 1.003 | ||
| O(26)-H(39) | 1.073 | ||
Table 1: Selected geometric parameters for N-Acryloyl-4-aminosalicylic acid (AAS) and its dimer form.
The HOMO–LUMO energy gap, ΔE, which is an important stability index, is applied to develop theoretical models for explaining the structure and conformation barriers in many molecular systems. The smaller is the value of ΔE, the more is the reactivity of the compound has [21,22,27]. The calculated quantum chemical parameters are given in Table 2.
| Comp. | EHOMO (a.u) | ELUMO (a.u) | ΔE (a.u) | χ (a.u) |
η (a.u) |
σ (a.u)-1 |
Pi (a.u) |
S (a.u)-1 |
ω (a.u) |
∆Nmax | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAS | -0.348 | -0.170 | 0.178 | 0.259 | 0.089 | 11.223 | -0.259 | 5.6117 | 0.3755 | 2.903 | ||||||||
| Dimer | -0.347 | -0.174 | 0.173 | 0.260 | 0.086 | 11.510 | -0.260 | 5.7554 | 0.3907 | 2.999 | ||||||||
Table 2 : Stabilization energy of AAS and its dimer, HOMO, LUMO and other additional parameters.
Additional parameters such as separation energies, ΔE, absolute electronegativities, χ, chemical potentials, Pi, absolute hardness, η, absolute softness, σ, global electrophilicity, ω, global softness, S, and additional electronic charge, ΔNmax, have been calculated according to the following equations (1-8) [21,22,28]:
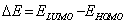 (1)
(1)
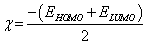 (2)
(2)
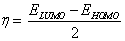 (3)
(3)
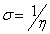 (4)
(4)
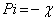 (5)
(5)
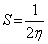 (6)
(6)
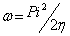 (7)
(7)
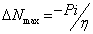 (8)
(8)
In the ring part, the CC bond lengths of the benzene rings are observed in the range 1.339 -1.35 Å. The CO bond lengths in the carboxylic acid group conform to the average values are tabulated for an aromatic carboxylic acid in which C=O is 1.229 Å and C-O is 1.349 Å. In AAS, C5-C4-C7 angle is smaller than C3-C4-C7 because of interaction between the carboxyl acid (COOH) and hydroxyl (OH) group (Figure 4A). These results are in agreement with literatures [23,29-31].
The torsional angles C5-C4-C7-O9 and C3-C4-C7-O8 are 178.8° and 179.3°, respectively. The tilt angles are calculated 180°. The dihedral angles are nearly the same among the all conformers. The geometric dimer structure of AAS is also calculated ( Figure 2). Stabilization energy lowest value of dimer showed higher stability than AAS. Interamolecular hydrogen bonds can be responsible for the geometry and the stability of a predominant conformation; the formation of hydrogen bonding between two molecules via a hydroxyl group and -COOH cause the structure of the dimer to be the most stable conformer (Figure 4B). The intermolecular hydrogen bonds are almost linear (the O–H…O angle equals 179.4°) and their length is 2.063 Å. The intramolecular hydrogen bonds between the hydroxyl groups and the oxygen atoms of the carbonyl groups are strongly bent (the O–H…O angle equals 164.7°) and the O…O distance is 2.056 Å [32].
Potentiometric studies
The interaction of a metal with an electron donor atom of a ligand (H2L) is usually followed by the release of H+. Alkaline potentiometric titrations are based on the detection of the protons released upon complexation. The main advantage of this technique, compared to other methods is that from the titration curves it is possible to follow complexation continuously as a function of pH and to detect exactly at which pH complexation takes place. Furthermore, it is possible to calculate the dissociation constants and the stability constants of its complexes from the potentiometric titration
The following equilibria were used for the determination of the pKa values of H2L (AAS) (eqs. 9 and 10) and the metal stability constants (eqs. 11 and 12):
H2L = HL- + H+ (9)
HL- = L2-+ H+ (10)
H2L + M2+ = ML + 2H+ (11)
H2L + ML = ML2 2- + 2H+(12)
Figures 5,6 shows a typical titration curve of the free acid in the absence and presence of compound AAS and its metal ion complexes. It can be seen that for the same volume of NaOH added, the compound titration curves show a lower pH value than the titration curve of free acid. From these titration curves, the average number of protons associated with N-Acryloyl-4-aminosalicylic acid molecule,nA, in monomeric (AAS) and polymeric (PAAS) forms were determined at different pH values applying the following Eq. 13:
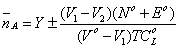 (13)
(13)
where Y is the number of available protons in AAS (Y=2) and V1 and V2 are the volumes of alkali required to reach the same pH on the titration curve of hydrochloric acid and reagent, respectively, V° is the initial volume (50 cm3) of the mixture, TC°L is the total concentration of the reagent, N° is the normality of the sodium hydroxide solution and E° is the initial concentration of the free acid. The titration curves
(nA vs. pH ) for the proton-ligand systems were constructed and found to extend between 0 and 2 on the nA scale (Figure 7). This means that AAS and PAAS have two dissociable protons (the hydrogen ion of the -OH phenolic moiety, pK1 H and the COOH group, pK2 H). Different computional methods [33] were applied to evaluate the dissociation constants. Three replicate titrations were performed; the average values obtained are listed in Table 3. The PAAS has a lower acidic character (higher pKH values) than AAS. This is quite reasonable because the presence of the vinyl group (H2C=CH) in monomeric form will decrease the electron density, whereby weaker O-H bond is formed. The absence of vinyl group in polymeric form will lead to the opposite effect (i.e., retard the removal of the ligand proton and hence increase the basicity of PAAS).
| Comp. | Temp. K |
Dissociation constant | Free energy change kJ mol-1 |
Enthalpy change kJ mol-1 |
Entropy change J mol-1 K-1 |
| pK1H pK2H | ΔG1 ΔG2 | ΔH1 ΔH2 | - ΔS1 - ΔS2 | ||
| AAS | 298 | 9.41 4.71 | 53.69 26.87 | 32.63 23.58 | 70.68 9.91 |
| 308 | 9.24 4.54 | 54.49 26.77 | 70.98 9.89 | ||
| 318 | 9.05 4.37 | 55.10 26.60 | 70.67 9.90 | ||
| PAAS | 298 | 9.89 5.00 | 56.43 29.44 | 29.88 24.50 | 89.48 13.52 |
| 308 | 9.72 4.86 | 57.32 29.36 | 90.25 13.51 | ||
| 318 | 9.64 4.73 | 58.69 29.28 | 87.94 13.53 |
Table 3: Thermodynamic functions for the dissociation of AAS and PAAS in 20 % (by volume) ethanol-water mixture in the presence of 0.1 M KCl at different temperatures.
The formation curves for the metal complexes were obtained by plotting the average number of the ligands AAS and PAAS attached per metal ions (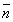 ) versus the free ligand exponent (pL), according to Irving and Rossotti [34]. The average number of the reagent molecules attached per metal ion,
) versus the free ligand exponent (pL), according to Irving and Rossotti [34]. The average number of the reagent molecules attached per metal ion,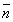 , and free ligand exponent, pL, can be calculated using the Eqs. 14 and 15:
, and free ligand exponent, pL, can be calculated using the Eqs. 14 and 15:
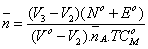 (14)
(14)
and
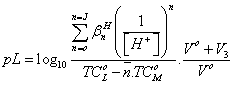 (15)
(15)
where TC°M is the total concentration of the metal ion present in the solution, βH n is the overall proton-reagent stability constant. V1, V2 and V3 are the volumes of alkali required to reach the same pH on the titration curves of hydrochloric acid, organic ligand and complex, respectively. These curves were analyzed and the successive stability constants were determined using different computional methods [35,36]. The values of the stability constants (log K1 and log K2) are given in Table 4.
| Mn+ | 298 K | 308 K | 318 K | ||||
| Comp. | log K1 | log K2 | log K1 | log K2 | log K1 | log K2 | |
| AAS | Mn2+ | 5.00 | 4.20 | 5.18 | 4.37 | 5.37 | 4.56 |
| Co2+ | 5.18 | 4.37 | 5.35 | 4.53 | 5.47 | 4.71 | |
| Ni2+ | 5.24 | 4.43 | 5.41 | 4.58 | 5.52 | 4.75 | |
| Cu2+ | 5.44 | 4.61 | 5.62 | 4.79 | 5.70 | 4.90 | |
| PAAS | Mn2+ | 7.44 | 6.41 | 7.67 | 6.63 | 7.89 | 6.82 |
| Co2+ | 7.63 | 6.59 | 7.85 | 6.78 | 8.06 | 6.98 | |
| Ni2+ | 7.72 | 6.65 | 7.91 | 6.86 | 8.13 | 7.07 | |
| Cu2+ | 7.95 | 6.87 | 8.14 | 7.07 | 8.35 | 7.26 | |
Table 4: Stepwise stability constants for ML and ML2 complexes of AAS and PAAS in 20 % (by volume) ethanol-water mixture in the presence of 0.1 M KCl at different temperatures.
The following general remarks can be made:
i) The maximum value of 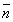 was ~ 2 indicating the formation of 1:1 and 1:2 (metal:ligand) complexes only [37].
was ~ 2 indicating the formation of 1:1 and 1:2 (metal:ligand) complexes only [37].
ii) The metal ion solution used in the present study was very dilute (2 x 10-5 M), hence there was no possibility of formation of metal hydroxide and polynuclear complexes [38,39].
iii) The metal titration curves were displaced to the right-hand side of the ligand titration curves along the volume axis, indicating proton release upon complex formation of the metal ion with the ligand. The large decrease in pH for the metal titration curves relative to ligand titration curves point to the formation of strong metal complexes [40,41].
iv) For all the complexes, the stability constants of PAAS are higher than AAS. This is quite reasonable because the ligand in polymeric forms are better complexing agent [15].
v) For the same ligand (AAS and PAAS) at constant temperature, the stability of the chelates increases in the order Mn2+, Co2+, Ni2+, Cu2+ [42 - 44]. This order largely reflects that the stability of Cu2+ complexes are considerably larger as compared to other metals of the 3d series. Under the influence of both the polarizing ability of the metal ion [45] and the ligand field [46], Cu2+ will receive some extra stabilization due to tetragonal distortion of octahedral symmetry in its complexes. The greater stability of Cu2+ complexes is produced by the well known Jahn–Teller effect [47].
Effect of temperature
The dissociation constants (pKH) for ASS and PAAS as well as the stability constants of its complexes with Mn2+, Co2+, Ni2+ and Cu2+ have been evaluated at (298, 308 and 318) K, and are given in Tables 3, 4. The enthalpy change (ΔH) for the dissociation and complexation process was calculated from the slope of the plot pKH or log K vs. 1/T using the graphical reperesentation of the van’t Hoff Eqs. 16 and 17:
ΔG = -2.303 RT log K = ΔH – T ΔS (16)
(or)
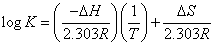 (17)
(17)
From the ΔG and ΔH values one can deduce the S using the well known relationships 16 and 18:
ΔS = (ΔH-ΔG) / T (18)
where R = 8.314 J K-1 mol-1is the gas constant, K is the dissociation constant for the ligand or the stability constant of the complex, and T is absolute temperature.
All thermodynamic parameters of the dissociation process of ASS and PAAS are recorded in Table 3. From these results the following conclusions can be made:
i) The pKH values decrease with increasing temperature, i.e., the acidity of the ligand increase.
ii) A positive value of ΔH indicates that the process is endothermic.
iii) A large positive value of ΔG indicates that the dissociation process is not spontaneous [45].
iv) A negative value of ΔS is obtained due to the increased order as a result of the solvation process.
All the thermodynamic parameters of the stepwise stability constants of AAS and PAAS complexes are recorded in Table 5.
| Comp. | Mn+ | T/K | free energy change (kJ mol-1) |
Enthalpy change ( kJ mol-1) |
Entropy change ( J mol-1 K-1) |
|||
| - ΔG1 | - ΔG2 | ΔH1 | ΔH2 | ΔS1 | ΔS2 | |||
| Mn2+ | 298 | 28.52 | 23.96 | 33.55 | 32.63 | 208.31 | 189.91 | |
| AAS | 308 | 30.54 | 25.77 | 208.11 | 189.61 | |||
| 318 | 32.69 | 27.76 | 208.32 | 189.92 | ||||
| Co2+ | 298 | 29.55 | 24.93 | 26.35 | 30.82 | 187.60 | 187.09 | |
| 308 | 31.55 | 26.71 | 187.98 | 186.80 | ||||
| 318 | 33.30 | 28.67 | 187.59 | 187.10 | ||||
| Ni2+ | 298 | 29.89 | 25.27 | 25.45 | 29.00 | 185.73 | 182.13 | |
| 308 | 31.90 | 27.00 | 186.21 | 181.84 | ||||
| 318 | 33.61 | 28.92 | 185.72 | 182.14 | ||||
| Cu2+ | 298 | 31.03 | 26.30 | 31.63 | 26.37 | 210.30 | 176.75 | |
| 308 | 33.14 | 28.24 | 210.31 | 177.33 | ||||
| 318 | 34.70 | 29.83 | 208.60 | 176.74 | ||||
| PAAS | Mn2+ | 298 | 42.45 | 36.57 | 40.82 | 37.21 | 279.43 | 247.59 |
| 308 | 45.23 | 39.09 | 279.39 | 247.75 | ||||
| 318 | 48.04 | 41.52 | 279.43 | 247.59 | ||||
| Co2+ | 298 | 43.53 | 37.60 | 39.01 | 35.36 | 276.99 | 244.83 | |
| 308 | 46.29 | 39.98 | 276.96 | 244.62 | ||||
| 318 | 49.07 | 42.49 | 276.99 | 244.84 | ||||
| Ni2+ | 298 | 44.04 | 37.94 | 35.36 | 30.01 | 266.47 | 228.03 | |
| 308 | 46.64 | 40.45 | 272.07 | 228.78 | ||||
| 318 | 49.50 | 43.04 | 272.49 | 229.74 | ||||
| Cu2+ | 298 | 45.36 | 39.19 | 36.26 | 35.38 | 273.89 | 250.26 | |
| 308 | 48.00 | 41.69 | 273.58 | 250.24 | ||||
| 318 | 50.84 | 44.20 | 273.90 | 250.26 | ||||
Table 5: Thermodynamic functions for ML and ML2 complexes of AAS and PAAS in 20 % (by volume) ethanol-water mixture and 0.1 M KCl.
It is known that the divalent metal ions exist in solution as octahedrally hydrated species [36] and the obtained values of ΔH and ΔS can then be considered as the sum of two contributions: (a) release of H2O molecules, and (b) metal-ligand bond formation. Examination of these values shows that:
i) The stepwise stability constants (log K1 and log K2) for AAS and PAAS complexes increases with increasing temperature [48].
ii) The negative value of ΔG for the complexation process of AAS and PAAS suggests the spontaneous nature of such processes [49,50].
iii) The ΔH values of AAS and PAAS are positive, meaning that these processes are endothermic and favourable at higher temperature.
iv) The ΔS values for the complexes of AAS and PAAS are positive, confirming that the complex formation is entropically favourable [14].
N-Acryloyl-4-aminosalicylic acid (AAS) has been synthesized and characterized using spectroscopic techniques ( IR and 1H NMR). The geometrical structures of these ligands are carried out by HF method with 3-21G basis set. The geometric dimer structure more sTable than AAS. The protonation constants (pK1and pK2) of the AAS and PAAS were determined by Irving-Rossetti pH titration technique. Also metal-ligand stability constants of their complexes with metal ions (Mn2+, Co2+, Ni2+ and Cu2+) have been determined potentiometrically. It appears that PAAS are better complexing agent with metal ions compaier d to AAS with the order: Mn(II) < Co(II) < Ni(II) < Cu(II) . The corresponding thermodynamic parameters (ΔG, ΔH and ΔS) were derived and discussed. The dissociation process is non-spontaneous, endothermic and entropically unfavorable. The formation of the metal complexes has been found to be spontaneous, endothermic and entropically favorable.