Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 1
Eye infection is caused by exposures to bacterial, fungal, viral and other microbial agents. Bacterial conjunctivitis is a common type of pink eye, caused by bacteria that infect the eye through various sources of contamination. The eye has several natural mechanisms to defend itself against infection or trauma. Medicinal plants frequently used as raw materials for extraction of active ingredients which is used in the synthesis of different drugs. Alternanthera sessilis (Linn) samples were collected from Tiruvannamalai District, Tamil Nadu, India. 2,2-diphenyl-1- picrylhydrazyl (DPPH) radical scavenging activity of stem extract showed better action than leaf extract. Ferric Reducing Antioxidant Power (FRAP) radical scavenging activity of leaf extract showed better action than stem extract. Antibacterial activity was screened for ocular disease-causing pathogens. The maximum zone of inhibition is found in Bacillus subtilis and lowest inhibition in Streptococcus mutans in leaf and stem extracts. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of leaf and stem extracts of Alternanthera sessilis (Linn) showed different concentrations with varying ocular pathogens. The Rabbit corneal cell line (SIRC) shows that there is no toxicity occurring in Normal cell line which clearly indicates that the MTT assay on Ocular cell line will inhibit the cytotoxic nature of the pathogen causing ocular diseases. Gas Chromatography Mass Spectrometry (GC - MS) and Fourier Transform Infra-Red Spectroscopy (FT-IR) analysis showed the presence of various compounds and functional group in the leaf and stem aqueous extract of Alternanthera sessilis (Linn).
Curcumin; Bioavailability; Conventional formulation; Nanocarrier; Microcarrier
Conjunctivitis is the cause of a red eye in patients which is a predominant feature of a serious eye condition, in which a delay in treatment due to a missed diagnosis can result in permanent visual loss. In addition, the inappropriate use of antibacterial topical eye preparations contributes to antimicrobial resistance. Most general practice clinics will not have access to specialized equipment for an eye examination, e.g., a slit lamp and tonometer for measuring intraocular pressure, and some conditions can only be diagnosed using these tools. Therefore, primary care management relies on noting key features such as pain, photophobia and reduced visual acuity, to identify which patient require referral for ophthalmological assessment. In general, a patient with a unilateral presentation of a red eye suggests a more serious cause than a bilateral presentation [1].
Bacterial conjunctivitis is usually caused by Streptococcus pneumoniae, Haemophilis influenzae, Staphylococcus aureus or Moraxella catarrhalis. Less commonly, Chlamydia trachomatis or Neisseria gonorrhoeae may be the causative organism. Symptoms are similar to viral conjunctivitis, but discharge is usually mucopurulent and may cause the eyelids to become “glued” together after sleeping. Bacterial conjunctivitis is self-limiting in most people and symptoms resolve without treatment within one to two weeks (although resolution may be more rapid in some people) [2].
Medicinal plants have a promising future because there are about half million plants around the world, and most of their medical activities have not been investigated yet, and their medical activities could be decisive in the treatment of present or future studies. Medicinal plants have many characteristics when used as a treatment, as Synergic medicine where the ingredients of plants all interact simultaneously, so their uses can complement or damage others or neutralize their possible negative effects, support of official medicines in the treatment of complex cases like cancer diseases the components of the plants proved to be very effective and in case of preventive medicine it has been proved that the component of the plants are also characterized by their ability to prevent the appearance of some diseases. This will help to reduce the use of the chemical remedies which will be used when the disease is already present i.e., reduce the side effect of synthetic treatment [3].
Alternanthera sessilis (Linn) is an annual or perennial herb, of 0.2 to 1 m high, with strong taproots. The leaves are simple, opposite, shortly petiolate or sessile, broadly lanceolate or spatulate of almost linear, 0.6 to 5 cm long, and 0.3 to 1 cm wide. They are attenuated at the base, and the apex is acute to blunt, with entire, globous or pilose (thin, fine, articulate hairs) margins. The stems are generally prostrate, creeping, often rooting at the nodes, sometimes floating or ascending at the tips, cylindrical and slightly hairy, with numerous, erect branches. The inflorescences are dense, sessile, silvery-white clusters of compressed spikes in the leaf axils; perianth segments are equal in length, acute, 1.5 to 2.5 mm long with a short point. Bracts are ovate, concave, 0.3 to 1 mm long and persistent; bracteoles are oblong-ovate, 1 to 1.5 mm long, may be acute, and not deeply lacerated. Sepals are 2 to 3 mm long, white or purplish, glossy with a green base, glabrous or with a few long hairs, and a strong midrib. The fruits are indehiscent, a small, flattened, obcordate or obovate utricle, 2 to 2.5 mm long, enclosing the seed. Seeds are dark-brown to black, disc-shaped and shiny, about 0.8 to 1 mm in diameter.
Collection and authentication of plant materials
The plant samples were collected from Tiruvannamalai, Tamil Nadu, India and it was identified as, Alternanthera sessilis (Linn) with the help of Flora of Presidency of Madras [4] and the flora of the Tamil Nadu and Carnatic [5]. The specimens were authenticated by Prof. P. Jayaraman, Plant Anatomical Research center, West Tambaram, Chennai, India.
Preparation of crude extract
The dried leaf and stem were powdered with the help of mechanical pulverizer. The powdered material was then soaked in 1000 mL of double distilled water. The extract was suction filtered using Whatmann filter paper. This was repeated for 2 to 3 times and similar extracts were pooled together and concentrated at 40°C to 45°C under reduced pressure using vacuum rotary evaporator. The concentrated crude aqueous extract was subjected to preliminary biological activities [6].
DPPH radical scavenging activity
The antioxidant (free radical scavenging) activity of the partitionates on the stable radical 1,1-diphenyl- 2-picrylhydrazyl (DPPH) was determined [7]. Here, 2.0 mg of each of the test sample was dissolved in methanol and solution of varying concentrations was obtained by serial dilution technique. Then 2 mL of each of the test sample was mixed with 3 mL of a DPPH methanol solution (20 μg/mL) and was allowed to stand for 20 min for the reaction to occur. The absorbance was determined at 517 nm and from these values, the corresponding percentage of inhibitions were calculated by using the following equation
% inhibition=[1-(ABSsample/ABScontrol)] × 100
Then % inhibitions were plotted against respective concentrations used and from the graph the IC50 was calculated using ascorbic acid, as the positive control. Three replicates of each sample were used for statistical analysis and the values are reported as mean ± SD.
Ferric reducing antioxidant power assay
Different concentrations of the aqueous extract of Alternanthera sessilis (Linn) and its various fractions (10 to 50 μg/mL) was added to 2.5 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide [K3Fe (CN)6] solution. The reaction mixture was vortexed well and then incubated at 50°C for 20 min using vortex shaker. At the end of the incubation, 2.5 mL of 10% trichloroacetic acid was added to the mixture and centrifuged at 3,000 rpm for 10 min. The supernatant (2.5 mL) was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1% ferric chloride. The colored solution was read at 700 nm against the blank with reference to standard using UV Spectrophotometer. Here, ascorbic acid was used as a reference standard, the reducing power of the samples were comparable with the reference standard [8].
Preparation of aqueous extract of Alternanthera sessilis (Linn)
The aqueous extract of wild plant of Alternanthera sessilis (Linn) was dried and dissolved with 100% Dimethyl sulfoxide (DMSO). The stock solutions were prepared as 10 mg/2 mL concentrations. From the stock solution 50 μL, 100 μL and 200 μL of sample extracts were immediately dispensed into each agar wells (9 mm) of culture inoculated Muller Hinton Agar (MHA) plates using sterilized micropipette.
Preparation of bacterial inoculums
Bacterial inoculum was prepared using 2 bacterial pathogens from a 24 h old culture on Muller Hinton Agar (MHA). With a sterile loop, the tops of four to five colonies were transferred to a tube containing 5 mL of Muller Hinton Broth (MHB). The tube was then incubated 37°C for 12 h. The turbidity of the culture suspension was adjusted with broth on a sterile saline solution (0.85 to 0.9%). The density of this culture was adjusted with 0.5 McFarland standards and finally made the inoculum size approximately of 5 × 105 CFU/mL.
Muller Hinton Agar
Muller Hinton Agar was purchased from HI Media laboratories Pvt. Ltd, Mumbai. 23.4 g of Muller Hinton Agar was suspended in 600 mL of distilled water in 1 L flask, stirred, boiled to dissolve and then autoclaved at 15 lbs and at 121°C for 15 min. The plates were poured in a sterile environment and covered. As soon as the agar was partially solidified, the plates were inverted and left aside for 2 h. Under aseptic conditions, the microorganisms were streaked onto the solidified plates and the wells were made by using 8 nm Cork borer that was sterilized with alcohol and flame. 10 mg of samples were dissolved in 2 mL of DMSO were pipette into the different wells at different volumes (50, 100, 200 μL) in separate plates using a micropipette for leaves and stem of Alternanthera sessilis (Linn) the plates were labelled and incubated at 37°C for 24 h for bacterial growth [9].
Percentage of inhibition (%)=[Zone of inhibition /Dia of the Petriplate (mm)] × 100
Rabbit Corneal Epithelial (SIRC) cells were cultured and maintained in 90% DMEM media substituted with 10% Foetal Bovine serum and 1% antibiotic for 48 h. The media was then removed, and the cell layer was washed with phosphate buffer saline PBS (0.1M pH 7.0). Later, 500 μl of trypsin-EDTA was added to the culture flask to remove the adherent cell layer from the flask. After 5 min, 2 mL of the media was added, and single cells were collected. The cells were counted on the haemocytometer to get the exact viability and cell count for the experiments.
Corneal (3 × 104) cells were seeded on 24- well plate and allowed to proliferate in 1 ml DMEM for 24 h. After 24 h, different concentrations of leaf and stem extract of Alternanthera sessilis (Linn) of 20 μgml-1, 40 μgml-1, 60 μgml-1, 80 μgml-1, and 100 μgml-1 were prepared from each extract and added to each well containing SIRC cells. After incubation with aqueous extracts for 24 h, MTT assay was performed to check the cytotoxic effect of various extracts on these cells to dissolve purple colour formazan crystals. The absorbance was observed at 490 nm in a spectrophotometer. Each experiment was performed in triplicates [10].
Extraction yields of extraction solvents
The leaf and stem sample of Alternanthera sessilis (Linn) extraction yielded percentages were obtained for the aqueous extract. Yield percentages were between 80-85% for the high polar solvent of aqueous extract. Ratio of 1:10 solid-liquid (Sample: Double distilled water) showed the highest yield percentage was obtained in aqueous solution. For better extraction yield 1:20 solid-liquid ratio was preferred.
Alternanthera sessilis (Linn) extraction yield percentages were obtained for different solvents. Yield percentages were between 44-78% for different solvents. For 1:10 solid-liquid ratio the highest yield percentage was obtained in aqueous solution. In 1:10 solid-liquid ratio for aqueous extraction yield percentage were clearly increased [11].
DPPH radical scavenging activity
Aqueous extracts of leaf and stem showed various ranges of percentage of inhibitions. Among the two different parts, stem extract showed maximum percentage of inhibition at 50 μg/mL followed by leaf extract 80 μg/mL whereas the standard Quercetin (60.14 μg/mL). The better antioxidant activity was observed in stem extract when compared with leaf (Table 1 and Graph 1).
| S. No | Conc (µg/mL) | % of inhibition | ||
|---|---|---|---|---|
| Leaf | Stem | Standard - Quercetin | ||
| 1 | 10 | 10.83 ± 0.015 | 25.15 ± 0.01 | 13.52 ± 0.00 |
| 2 | 20 | 23.21 ± 0.190 | 39.94 ± 3.05 | 23.33 ± 0.01 |
| 3 | 30 | 23.79 ± 0.026 | 42.38 ± 1.37 | 39.21 ± 0.02 |
| 4 | 40 | 26.83 ± 0.015 | 48.02 ± 0.03 | 41.96 ± 0.01 |
| 5 | 50 | 37.7 ± 0.010 | 49.84 ± 0.04 | 47.45 ± 0.00 |
| 6 | 60 | 44.56 ± 0.026 | 53.04 ± 0.03 | 48.82 ± 0.00 |
| 7 | 70 | 45.72 ± 0.010 | 53.45 ± 0.02 | 53.33 ± 0.00 |
| 8 | 80 | 49.88 ± 0.015 | 55.71 ± 0.02 | 57.64 ± 0.00 |
| 9 | 90 | 53.71 ± 0.01 | 56.11 ± 0.10 | 59.41 ± 0.00 |
| 10 | 100 | 67.99 ± 0.015 | 60.38 ± 0.28 | 72.94 ± 0.00 |
Table 1: DPPH Antioxidant activity of Alternanthera sessilis Linn.
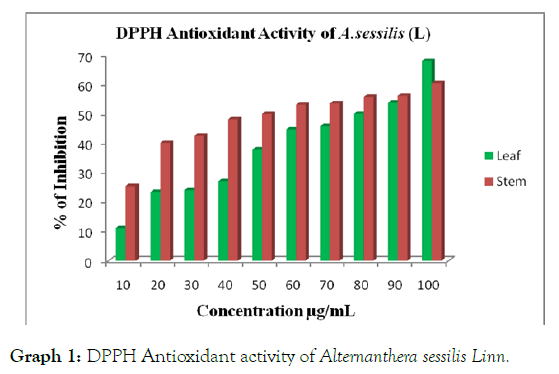
Graph 1: DPPH Antioxidant activity of Alternanthera sessilis Linn.
Any oxidant entering the biological system can maximally manifest its damaging effects only after entry into the cellular environment and by passing the endogenous antioxidant system. For the entry into the cell or its organelles, an oxidant needs to cross the membrane barriers both plasma membrane and internal membranes [12].
Total antioxidant activity in aqueous leaf and stem extract of Alternanthera sessilis (Linn) was determined by FRAP assay. In this evaluation, the antioxidant activity is evaluated based on the capacity to reduce Ferric (III) iron to Ferrous (II) iron. The result demonstrates that the stem extracts possess higher antioxidant capacity compared to the standards used which is Quercetin. FRAP value of the stem extract is >50 μg/mL followed by the FRAP standard value of the quercetin is >60 μg/mL and leaf extract shows >100 μg/mL which clearly indicates that the stem extract showed good antioxidant activity compared to the leaf extract (Table 2 and Graph 2).
| S. No | Conc (µg/mL) | % of inhibition | ||
|---|---|---|---|---|
| Leaf | Stem | Standard - Quercetin | ||
| 1 | 10 | 15.23 ± 1.01 | 25.48 ± 0.57 | 13.52 ± 0.00 |
| 2 | 20 | 18.57 ± 2.11 | 39.61 ± 2.79 | 23.33 ± 0.01 |
| 3 | 30 | 20.38 ± 0.49 | 42.04 ± 0.86 | 39.21 ± 0.02 |
| 4 | 40 | 23.41 ± 0.69 | 47.69 ± 0.58 | 41.96 ± 0.01 |
| 5 | 50 | 24.95 ± 0.16 | 49.51 ± 0.54 | 47.45 ± 0.00 |
| 6 | 60 | 27.42 ± 0.06 | 52.71 ± 0.60 | 48.82 ± 0.00 |
| 7 | 70 | 31.57 ± 0.54 | 53.78 ± 0.55 | 53.33 ± 0.00 |
| 8 | 80 | 35.45 ± 1.06 | 56.04 ± 0.55 | 57.64 ± 0.00 |
| 9 | 90 | 39.53 ± 1.06 | 56.78 ± 1.05 | 59.41 ± 0.00 |
| 10 | 100 | 43.02 ± 0.05 | 60.38 ± 0.28 | 72.94 ± 0.00 |
Table 2: FRAP Antioxidant activity of Alternanthera sessilis Linn.
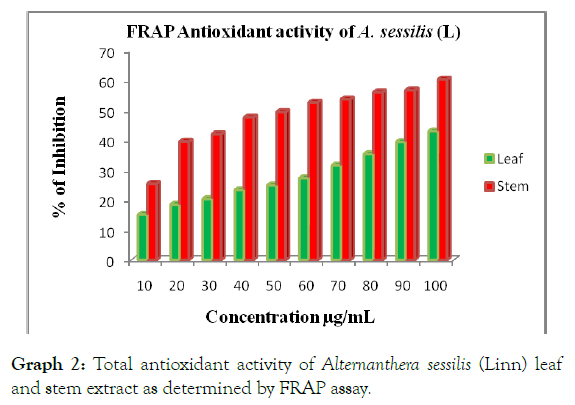
Graph 2: Total antioxidant activity of Alternanthera sessilis (Linn) leaf and stem extract as determined by FRAP assay.
Leaves of A. sessilis had more FRAP values than the A. tristis . A. sessilis showed more radical scavenging activity and FRAP values, which may be due to the accumulation of flavonoids and other phenolics that are directly proportional to the free radical scavenging activity of the extracts. FRAP assay was used to evaluate the reducing power of several other fruits and vegetables like tomato cultivars and in African, leafy vegetables [13-15].
Alternanthera sessilis (Linn) is blended into equal proportion of leaf and stem aqueous extracts. It was tested for its potency as an antimicrobial. The aqueous extracts of Alternanthera sessilis (Linn) were subjected to antimicrobial study (agar well diffusion method) and the results obtained were measured to nearest millimetre (mm) and recorded in terms of its IZD values. Aqueous extract of Alternanthera sessilis (Linn) leaf was found to be most effective against the chosen microbes. The leaf extract of Alternanthera sessilis (Linn) was found to be the most effective against Bacillus subtilis, Staphyllococcus aureus, and Streptococcus mutans showing a zone of inhibition (ZOI) of 25 ± 27.77 mm, 20 ± 22.22 mm and 19 ± 21.11 mm at a concentration of 1mg/100μl, whereas the stem extract of Alternanthera sessilis (Linn) was tested against Bacillus subtilis, Staphyllococcus aureus, and Streptococcus mutans, ZOI was determined to be 24 ± 26.66 mm, 20 ± 22.22mm and 12 ± 13.33 mm respectively. All microbes were found highly susceptible to reference antibiotic. Kanamycin being a pure antibiotic inhibited all bacteria in reference giving highest ZOI of inhibition than the extracts of Alternanthera sessilis (Linn) (Tables 3 and 4).
| Concentration of aqueous extracts of Alternanthera sessilis (Linn) leaf Alternanthera sessilis | ||
|---|---|---|
| Organisms | Zone of Inhibition (mm) | % of inhibition |
| Bacillus subtilis | 25 | 27.77 |
| Staphylococcus aureus | 20 | 22.22 |
| Streptococcus mutans | 19 | 21.11 |
Table 3: Antibacterial activity of Alternanthera sessilis (Linn) leaf extract against pathogens causing ocular diseases.
| Concentration of aqueous extracts of Alternanthera sessilis (Linn) stem Alternanthera sessilis | ||
|---|---|---|
| Organisms | Zone of Inhibition (mm) | % of inhibition |
| Bacillus subtilis | 24 | 26.66 |
| Staphylococcus aureus | 20 | 22.22 |
| Streptococcus mutans | 12 | 13.33 |
Table 4: Antibacterial activity of Alternanthera sessilis (Linn) stem extract against pathogens causing ocular diseases.
The minimal inhibitory concentration required for inhibiting the growth of the ocular pathogens and the concentration of extract obtained to be bactericidal was found to vary with different organisms (Tables 5 and 6). The experiments were performed in triplicates with calculated standard deviation that was found to be insignificant in all the cases in MIC studies.
| Organisms | MIC, MBC and MIC Index of aqueous leaf extracts of Alternanthera sessilis (Linn) in mg/mL |
||
|---|---|---|---|
| Minimum Inhibitory concentration (MIC) | Minimal Bactercidal Concentration (MBC) | MIC Index | |
| Bacillus subtilis | 0.25 | 0.5 | 2 |
| Staphylococcus aureus | 0.063 | 0.125 | 2 |
| Streptococcus mutans | 0.125 | 0.25 | 2 |
Table 5: Represents the Minimal Inhibitory Concentration (MIC) and Minimal Bactercidal Concentration (MBC) of aqueous leaf extract of Alternanthera sessilis (Linn).
| Organisms | MIC, MBC and MIC Index of aqueous extracts of Alternanthera sessilis (Linn) in mg/mL | ||
|---|---|---|---|
| Minimum Inhibitory concentration (MIC) | Minimal Bactercidal Concentration (MBC) | MIC Index | |
| Bacillus subtilis | 0.125 | 0.25 | 2 |
| Staphylococcus aureus | 0.063 | 0.125 | 2 |
| Streptococcus mutans | 0.063 | 0.125 | 2 |
Table 6: Represents the Minimal Inhibitory Concentration (MIC) and Minimal Bactercidal Concentration (MBC) of aqueous stem extract of Alternanthera sessilis (Linn).
The triphala act as an effective drug for eye diseases caused either by microbes in reference, due to excess of free radical production or due to various physiological activities affecting the normal functioning of the eye. The ZOI and MIC values obtained for various extracts shows triphala to possess phytosignatures which are responsible for scavenging the growth of the microbes [16].
The effect of aqueous extracts of Alternanthera sessilis (Linn) on the metabolic activity of corneal cells (SIRC) evaluated using MTT assay demonstrates that the metabolic activity of cells was not significantly comprised thereby aqueous extracts is not cytotoxic in nature. The growth of the cells at the varied concentration range (20 μg/mL to 100 μg/mL) was found to enhance leaf or stem extracts to be equivalent (corneal cells). Leaf and stem extracts shows slightly higher metabolic rate from 20 μg/mL to 100 μg/mL whereas, the metabolic rate of the SIRC cells were slightly affected in presence of leaf and stem extract of Alternanthera sessilis (Linn) with increase in concentration of the extracts as compared to the untreated corneal cells as shown in Figure 1. The treatment of SIRC under the applied conditions at different doses (20 μg/mL to 100 μg/mL of media) registered a fall in the cell count after 24 h of incubation unproportionate to the dose applied exhibiting non-cytotoxic nature of leaf and stem extracts coinciding with the incremental increase (20 μg/mL of doses of these extracts). The leaf extract has indicated its cytoprotective nature as with the increase of dose, the cell count increased at the rate of 13%, (20 μg/ mL), 23% (40 μg/mL), 40% (60 μg/mL), 60% (80 μg/mL) and 80% (100 μg/mL) than control cells. The stem extract also exhibited cytoprotectivity where the cell count showed an appreciation of 20% increase at 20 μg/mL but with increase in concentration the percentage increase was 35% (40 μg/mL), 50% (60 μg/mL), 74% (80 μg/mL) and 86% (100 μg/mL) but more than the initially seeded cell count. The microscopic images showed the normal cell morphology (Figure 2).
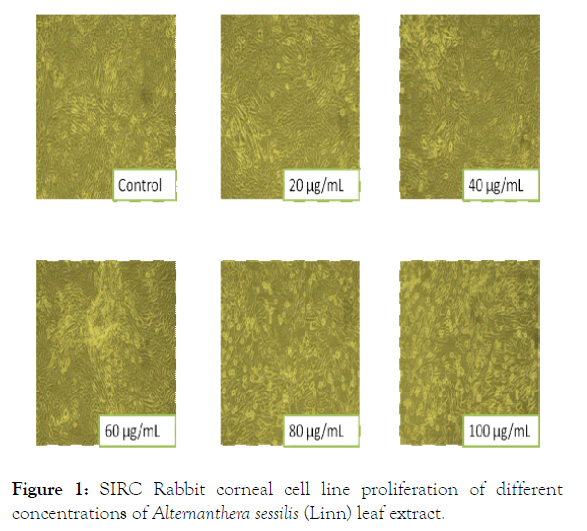
Figure 1: SIRC Rabbit corneal cell line proliferation of different concentrations of Alternanthera sessilis (Linn) leaf extract.
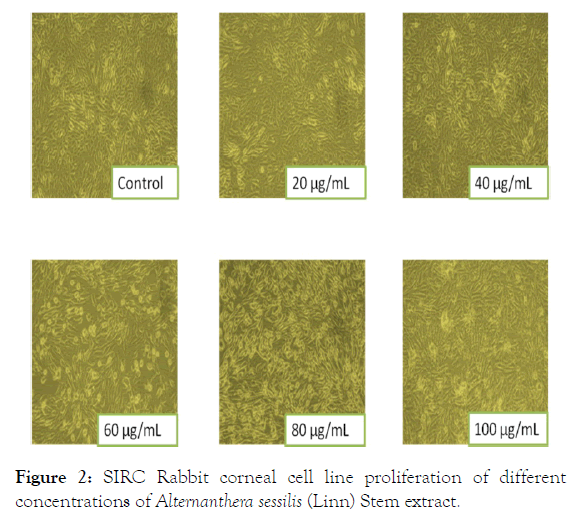
Figure 2: SIRC Rabbit corneal cell line proliferation of different concentrations of Alternanthera sessilis (Linn) Stem extract.
MTT assay (which is the measure of absorbance of end product i.e., formazan crystals, reflecting the metabolic activity of cells), the S2 extract shows to support higher cell proliferation with enhanced metabolic activity, thereby it has given indication of cytoprotective nature of extract. The cells in the presence of media (defined and described earlier) were incubated for 24 h to achieve 70% confluency of cells. The confluent cells were treated with 10 μg/mL to 50 μg/mL of media of all extracts and were further incubated for 24 h [16].
The GC- MS analysis of aqueous extract of Alternanthera sessilis (Linn) leaf showed the presence of Undecanoic acid, 10-methyl-, methyl ester, 4H-1-Benzopyran-4-one, 7-hydroxy-2-phenyl, Phytol, (E )-9-Octadecenoic acid ethyl ester, 14-Hydroxy-15- methylhexadec-15-enoic acid, ethyl ester, Elaidic acid, isopropyl ester, 9,12,15-octadecatrienoic acid 2,3-dihydroxypropyl ester, (Z,Z,Z ), Propylene glycol monooleate, Docosanoic acid, methyl ester, 9,9'- Biphenanthrene, octacosahydro, Lup-20(29)-en-3-one (Table 7 and Graph 3) and the stem extract of Alternanthera sessilis (Linn) showed the presence of 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4 hydroxyphenyl), Oleic acid, (E )-9-Octadecenoic acid ethyl ester, 11- Eicosenoic acid, methyl ester, Pyrrolidine, 1-(1-oxo-2,5- octadecadienyl), Heneicosanoic acid, 20-oxo-, methyl ester (Table 8 and Graph 4).
| Retention time | Compound | Chemical formula | Molecular weight |
|---|---|---|---|
| 17.15 | Undecanoic acid, 10-methyl-, methyl ester | C13H26O2 | 214.344 |
| 17.8 | 4H-1-Benzopyran-4-one, 7-hydroxy-2-phenyl- | C15H10O3 | 238.242 |
| 18.83 | Phytol | C20H40O | 296.539 |
| 19.45 | (E)-9-Octadecenoic acid ethyl ester | C20H38O2 | 310.515 |
| 19.68 | 14-Hydroxy-15-methylhexadec-15-enoic acid, ethyl ester; | C19H36O3 | 312.494 |
| 20.32 | Elaidic acid, isopropyl ester | C21H40 | 324.541 |
| 21.47 | 9,12,15-octadecatrienoic acid ,2,3-dihydroxypropyl ester, (Z,Z,Z) | C21H36O4 | 352.515 |
| 22.47 | Propylene glycol monooleate | C21H40O3 | 340.548 |
| 24.05 | Docosanoic acid, methyl ester | C23H46O2 | 354.61 |
| 26.02 | 9,9'-Biphenanthrene, octacosahydro- | C28H46 | 382.676 |
| 33.75 | Lup-20(29)-en-3-one | C30H48O | 424.713 |
Table 7: GC-MS spectrum of aqueous extract of Alternanthera sessilis (Linn) leaf.
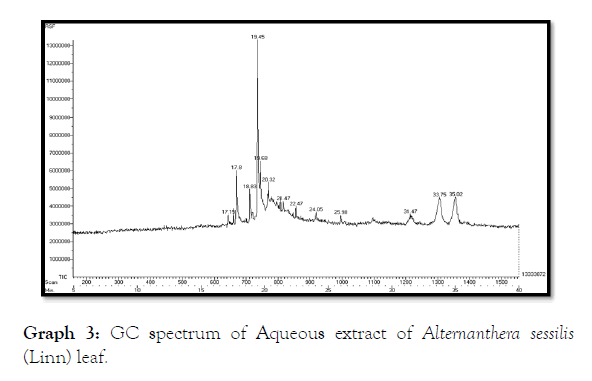
Graph 3: GC spectrum of Aqueous extract of Alternanthera sessilis (Linn) leaf.
| Retention time | Compound | Chemical formula | Molecular weight |
|---|---|---|---|
| 17.85 | 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4 hydroxyphenyl) | C15H10 | 270.237 |
| 18.98 | Oleic acid | C18H34O2 | 282.468 |
| 19.48 | (E)-9-Octadecenoic acid ethyl ester | C20H38O2 | 310.515 |
| 20.98 | 11-Eicosenoic acid, methyl ester | C21H40O2 | 324.541 |
| 21.7 | Pyrrolidine, 1-(1-oxo-2,5-octadecadienyl)- | C22H39NO | 333.551 |
| 22.45 | Heneicosanoic acid, 20-oxo-, methyl ester | C22H42O3 | 354.567 |
Table 8: GC-MS spectrum of aqueous extract of Alternanthera sessilis (Linn) stem.
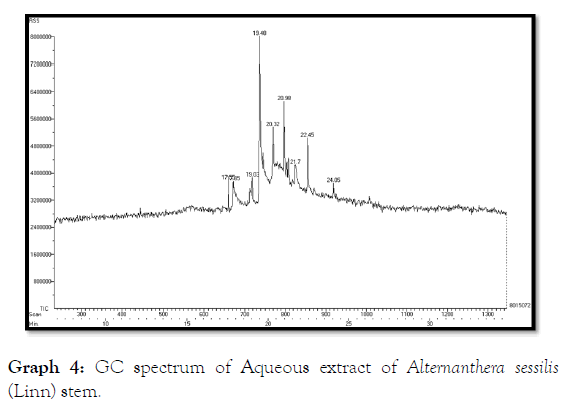
Graph 4: GC spectrum of Aqueous extract of Alternanthera sessilis (Linn) stem.
The various retention time of Alternanthera sessilis (Linn) leaf from 2.03 to 46.10 possessing different compounds which showed the presence of oxygenated sesquiterpenes, diterpenes, ketones, fatty acid and esters [17]. The stem extract of Alternanthera sessilis (Linn) showed retention time varying from 2.02 to 45.82 having potential compounds as a free radical scavenging property. Thus, the study on Antioxidant and Antibacterial effect on Alternanthera sessilis (Linn) proved to be an essential plant which can be effectively used as drug and purification of drug can be formulated against ocular diseases [18].
The volatile products can be found in the spectrum of Alternanthera sessilis (Linn) leaf and stem extracts. The leaf extract shows peaks between 604 and 3395 cm-1, showing different functional groups such as alcohols at 3395 cm-1, Phenols at 3395 cm-1, 1° amines at 3395 cm-1, 1645 cm-1 and 887 cm-1, 2° amines at 3395 cm-1 and 887 cm-1, amides at 3395 cm-1and 887 cm-1, aromatics at 3011 cm-1, 1461 cm-1, 1408 cm-1 and 887 cm-1, alkenes are present at 3011 cm-1 and 1645 cm-1, Alkanes at 2924 cm-1, 2853 cm-1, 1461 cm-1 and 721 cm-1, esters at 1736 cm-1, Saturated aliphatic at 1736 cm-1, aldehydes at 1736 cm-1, alkyl halides 1167 cm-1, 721 cm-1 and 604 cm-1 and the aliphatic amines are found in peaks of 1167 cm-1 and 1075 cm-1 (Table 9 and Graph 5).
| Frequency | Bond | Functional group |
|---|---|---|
| 3395 cm-1 | O-H stretch, H- bonded, N-H Stretch | alcohols, Phenols, 1°, 2° amines, amides |
| 3011 cm-1 | =C-H, =C-H stretch | aromatics, alkenes |
| 2924 cm-1 | C-H Stretch | Alkanes |
| 2853 cm-1 | C-H Stretch | Alkanes |
| 1736 cm-1 | C=O Stretch | esters, Saturated aliphatic, aldehydes, saturated aliphatic |
| 1645 cm-1 | -C=C – Stretch, N-H bend | alkenes, 1° amines |
| 1461 cm-1 | C-H bend, C-C stretch (in ring) | alkanes, aromatics |
| 1408 cm-1 | C-C Stretch (in-ring) | Aromatics |
| 1167 cm-1 | C-H wag t- CH2X1, C-N Stretch | alkyl halides, aliphatic amines |
| 1075 cm-1 | C-N Stretch | aliphatic amines |
| 887 cm-1 | N-H wag, C-H “oop” | 1°, 2° amines, amides, aromatics |
| 721 cm-1 | C-Cl stretch, C-H rock | alkyl halides, alkanes |
| 604 cm-1 | C-Cl stretch, C-Br Stretch | alkyl halides |
Table 9: FT - IR spectrum of aqueous extract of Alternanthera sessilis (Linn) leaf.
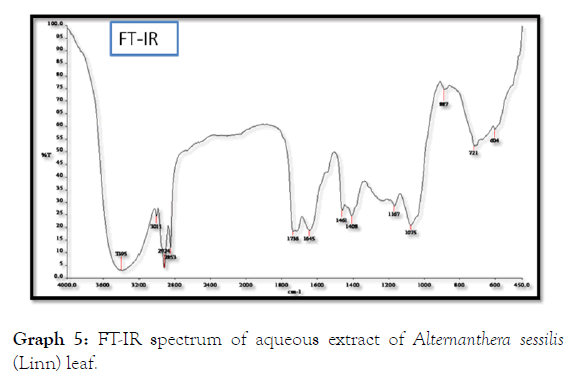
Graph 5: FT-IR spectrum of aqueous extract of Alternanthera sessilis (Linn) leaf.
The stem extract of Alternanthera sessilis (Linn) showed bands between 490 to 3360 cm-1 showing functional groups 1°, 2° amines at 3360 cm-1 and 1648 cm-1, amides at 3360 cm-1, Alkanes at 2972 cm-1, 2658 cm-1, 1466 cm-1, 1380 cm-1 and 649 cm-1, Nitro compounds are present at 1341 cm-1, Aromatic amines are found at 1306 cm-1, Alkyl halides at 1161 cm-1, Alcohols carboxylic acids, esters, ethers are present at 1129 cm-1, 1001 cm-1 and Alkenes at 952 cm-1, 817 cm-1 and 777 cm-1 (Table 10 and Graph 6).
| Peaks | Bond | Functional group |
|---|---|---|
| 3360 cm-1 | N-H | 1°,2° amines, amides |
| 2972, 2658 cm-1 | C-H | Alkanes |
| 1648 cm-1 | N-H | 1 amine |
| 1466, 1380 cm-1 | C-H | Alkanes |
| 1341 cm-1 | N-O | Nitro compounds |
| 1306 cm-1 | C-N | Aromatic amines |
| 1161 cm-1 | C-H-CH2X | Alkyl halides |
| 1129, 1001 cm-1 | C-O | Alcohols carboxylic acids, esters, ethers |
| 952 cm-1 | =C-H | Alkenes |
| 817, 777 cm-1 | C-H | Alkenes |
| 649 cm-1 | =C-H | Alkanes |
| 491 cm-1 | C-Br | Alkyl halides |
Table 10: FT-IR spectrum of aqueous extract of Alternanthera sessilis (Linn) stem.
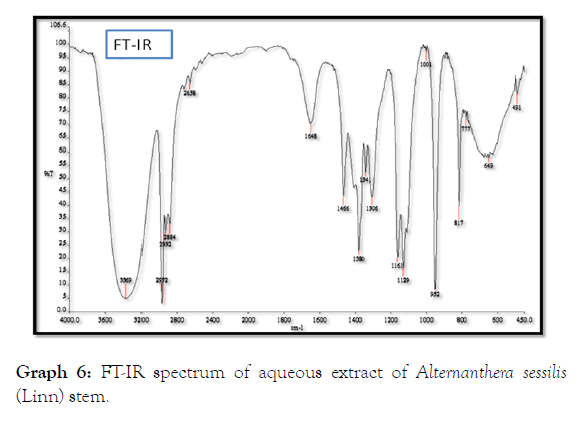
Graph 6: FT-IR spectrum of aqueous extract of Alternanthera sessilis (Linn) stem.
Similarly Ashe [19] reported that the Fourier transformation infrared (FTIR) spectrum of the leaf and stem extract of Alternanthera sessilis (Linn) and Achyranthus aspera showed numerous functional groups probably responsible for the inhibition of biofilm. The FTIR spectra of silver nanoparticles synthesized using aqueous extract of Alternanthera sessilis the peaks located at 3,253 and 1,634 cm-1 may be due to the presence of −NH or −OH group and carbonyl stretching in proteins. The other peaks at 2,190 and 2,040 cm-1 are assigned to C≡C or C≡N respectively [20].
The secondary metabolites of Alternanthera sessilis (Linn) have been found as free radical scavengers and bactericides that viably suites to manage and contain the oxidative stress and bacteria that are directing ophthalmic disorder(s) in vitro and are safely extendable for the in vivo studies. It further opens the area for exploring the active phytosignatures present in herbal plants to be used as a growth rejenuvator or for obtaining the growth regulatory compounds. These plant-based formulations with zero side effects are safer clinically than the steroid and penicillin-based eye drop formulation with various side effects.
We thank the Head, Department of Biotechnology, University of Madras, Chennai for his support and encouragement and Co- Ordinator, DST - PURSE Program, University of Madras, Chennai for providing the financial help during the course of study.
Citation: Suganya D, Banupriya R, Uma maheswari A, Elumalai S (2019) Studies on Biological Activity of Aqueous Extract of Alternanthera sessilis (Linn) for Developing Potential Herbal Drug Formulation of Ocular Diseases. Med Aromat Plants (Los Angeles) 8:327. doi: 10.35248/2167-0412.19.8.327
Received: 05-Dec-2018 Accepted: 26-Dec-2018 Published: 03-Jan-2019 , DOI: 10.35248/2167-0412.19.8.327
Copyright: © 2019 Suganya D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.