Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2016) Volume 7, Issue 1
Mixed Formula is the main form and method of Traditional Chinese Medicine (TCM) treatment. It can produce different therapeutic effects by changing the combination of TCM Formula. Wuji Wan is a formula of TCM and was composed of Rhizoma Coptidis (Huanglian in Chinese, HL), Fructus Evodiae Rutaecarpae (Wuzhuyu, WZY) and Radix Paeoniae Alba (Baishao, BS). Wuji Wan is mainly used for the treatment of intestinal diseases. This paper took Wuji Wan as example study on efficacy Mechanism of TCM Combination Based on drug metabolizing Enzyme. Inhibition of cytochrome P450 (CYP) is regarded as the most clinically important pharmacokinetic causes among the various possible factors for drug-drug or herb-herb/herb-drug interactions. In this study, the in vitro inhibitory effects of Wuji Wan with different combination within HL, WZY and BS on six major rat CYPs (CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A1/3A2) activities were examined by using HPLC and LC-MS. Wuji Wan with different combination were designed as 9 Formulae according to orthogonal table L9 (34); meanwhile the inhibitory effects of the single herb HL, WZY and BS also were done and compared with 9 Wuji Wan Formulae with different combination. Results demonstrated that BS showed negligible inhibitory effects on the six major CYP isoenzymes in rat liver microsomes, but HL showed strong inhibitory effects on 6 CYPs with almost all of the IC50 values below 200 μg (crude drug)•mL-1, and WZY showed a little bit inhibitory effects on 6 CYPs with the IC50 values between 870-2000 μg (crude drug)•mL-1; moreover, 9 Wuji Wan Formulae showed different inhibitory characteristic following with the dose levels of HL, WZY and BS in L9(34) design. In conclusion, this study demonstrates that Wuji Wan is likely to cause significant herb-drug interactions in humans when co-administered with substrates of the six CYPs (CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A)and also the different inhibitory effects on cytochrome P450 of Wuji Wan within different combination could be one of the reasons for explaining the different combination of TCM Formula producing different therapeutic effects, because the chemical composition of TCM in vivo (prototype or metabolites) is the Material basis of TCM effects.
Keywords: TCM Formula; Rhizoma coptidis; Fructus evodiae Rutaecarpae; Radix paeoniae Alba; Cytochrome P450; Herb-drug interactions
Mixed Formula is the main form and method of Traditional Chinese Medicine (TCM) treatment [1]. It can produce different therapeutic effects by changing the combination of TCM Formula. Due to complex ingredients and varietal actions, it is difficult to explain the efficacy mechanism of TCM Formula. A logical explanation to the development of mixed formula was that healers wanted to enhance effects, and at the same time reduce toxicity which, theoretically, could be lowered by adding antidotic herbs. However, the wisdom behind the Formulae was a lot more sophisticated than the consideration of efficacy enhancement and detoxication [2,3]. A formula was constructed for efficacy, support, safety and preparation for other directions of achievement (å??è?£ä½?使, sovereign, minister, assistant and courier) [4,5]. The formula is created not only for the control of symptoms but also for a transition to health promotion. Also because the most of TCM Formulae are created by healers through clinical experiences without scrupulous and comparable experimental data, now it is more of a hindrance than a help for the development and usage of TCM [6]. It is realized that the more mechanisms of TCM Formulae action are elucidated, the more advantages can be utilized in modern medical practice. Facing the fact that there is a genuine rising respect and need for alternative medicine, both the service providers and the users, should seriously consider the status of scientific utilization in alternative medicine [7]. The ultimate aim of this exercise is that eventually, an intelligent use of the traditional practice of cure can be integrated into modern scientific practice, in a consensual need to bring improved health and well-being to the majority of patients [8]. Nowadays, more and more TCM practicers and researchers address themselves to uncovering the mysteries of TCM Formula combination.
Our research team has been working on the compatibility mechanism of Wuji Wan [9-12]. Wuji Wan is a formula of TCM and was composed of Rhizoma Coptidis (Huanglian in Chinese, HL), Fructus Evodiae Rutaecarpae (Wuzhuyu, WZY) and Radix Paeoniae Alba (Baishao, BS). Wuji Wan is mainly used for the treatment of gastrointestinal disorders [13-15]. There were studies showed that Wuji Wan has a different efficacy characteristics if treat with different combination of HL, WZY and BS [16-18]. In ancient books on medicine, it is described that the prescription and dose of Wuji Wan varies with the individual ‘‘syndrome’’ (Zhenghou in Chinese). For example, Wuji Wan (HL/WZY/BS proportion is 5:5:5) as described in the New Book of Pediatrics and Formulary of the Bureau of Taiping People’s Welfare Pharmacy in Song Dynasty could provide relief from pouring diarrhea and abdominal pain, and other Formulae (10:2:2 and 6:1:6), described in Investigations of Medical Prescriptions and Chinese Pharmacopeia, help abate flatulence and acid regurgitation [19].
Cytochrome P450 (CYP), a superfamily of monooxygenases located primarily in hepatocytes, are the enzymes principally responsible for the metabolism of a lot of endogenous and exogenous compounds [20]. It is generally accepted that 80%–90% of the clinical drugs are metabolized by CYP isoenzymes [21,22]. Of these, CYP3A accounts for approximately 30.2%, CYP2D6 for 20%, CYP2A6 for 3.8%, CYP2E1 for 5.4%, CYP2C19 for 6.8% and CYP1A2 for 8.9% [23,24]. Alteration of CYP activities involved in the absorption, distribution, metabolism, or excretion of a drug maybe change drug exposure and affect drug response (safety or efficacy) [25,26]. In this study, we want to investigate the effects of Wuji Wan compound with different combination on the levels of enzymic activity of CYPs (CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A1/3A2) in rat liver microsomes in vitro, and to explain the compatibility mechanism of Wuji Wan from the point of relationships between Compound Prescription of TCM and Metabolism. It is our hypothesis that different combination of TCM Formula producing different therapeutic effects is associated with metabolic characteristics of chemical composition because the chemical composition of TCM in vivo (prototype or metabolites) is the Material basis of TCM effects. So we want to take Wuji Wan as an example for probing the TCM Formula compatibility mechanism from the relationship of “TCM compatibility - Metabolism”.
In this study, the in vitro inhibitory effects of Wuji Wan with different combination within HL, WZY and BS on six major rat CYPs (CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A1/3A2) activities were examined by using HPLC and LC-MS [20,27]. Wuji Wan with different combination were designed as 9 Formulae according to orthogonal table L9(34); meanwhile the inhibitory effects of the single herb HL, WZY and BS also were done and compared with 9 Wuji Wan Formulae with different combination. It is expected that the results will be useful to evaluate the relationship between the bioavailabilities based on metabolism and prescription proportion of Wuji Wan, finally providing guidance for the clinical application of traditional Chinese medical formula.
The substrates/probe drug and inhibitors of CYPs used in this study were in line with the FDA’s guideline and previous reports [28-31]. These experimental methods have been validated in our previous study [32,33], and IC50 values of inhibitors were in good agreement with the published values according to the acceptable degree of accuracy. 9 Wuji Wan Formulae raised from orthogonal table L9(34) and 3 single herbs (HL, WZY and BS) were evaluated for the ability to inhibit the activities of the six CYPs (CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A1/3A2). The IC50 values for six CYPs in rat liver microsomes are presented in Table 2; the Formulae ratios of Wuji Wan and concentration are shown in Table 1.
| Factor, Level and drug compatibility ratios | |||||
|---|---|---|---|---|---|
| Conditions serial number | A Factor: RhizomaCoptidis(HL) Herb concentration/μg (crude drug)•mL-1 |
B Factor: FructusEvodiaeRutaecarpae(WZY) Herb concentration/μg (crude drug)•mL-1 |
C Factor: Radix Paeoniae Alba(BS) Herb concentration/μg (crude drug)•mL-1 |
D Factor: 0 | A:B:C Ratios Herb concentration/μg (crude drug)•mL-1 |
| 1#formula (1#F) | 3/168 | 1/56 | 3/168 | - | 3: 1: 3/392 |
| 2# formula (2#F) | 3/168 | 2/112 | 6/336 | - | 3: 2: 6/616 |
| 3# formula (3#F) | 3/168 | 6/336 | 12/672 | - | 3: 6: 12/1176 |
| 4# formula (4#F) (adopted by China Pharmacopoeia) | 6/336 | 1/56 | 6/336 | - | 6: 1: 6/728 |
| 5# formula (5#F) | 6/336 | 2/112 | 12/672 | - | 6: 2: 12/1120 |
| 6# formula (6#F) | 6/336 | 6/336 | 3/168 | - | 6: 6: 3/840 |
| 7# formula (7#F) | 12/672 | 1/56 | 12/672 | - | 12: 1: 12/1400 |
| 8# formula (8#F) | 12/672 | 2/112 | 3/168 | - | 12: 2: 3/952 |
| 9# formula (9#F) | 12/672 | 6/336 | 6/336 | - | 12: 6: 6/1344 |
| single HL | 672.24 | 672.24 | |||
| single WZY | 4259.32 | 4259.32 | |||
| single BS | 35870 | 35870 | |||
Note:
1 All Wuji Wan Formulae and single HL, WZY, BS was prepared in extract, but the dose/concentration was expressed as crude drug.
2 The gradient concentration for detecting IC50 of Wuji Wan on six major CYPs begins at concentrations of Table 1 and dilutes to half concentrations continually until getting 6 gradient concentrations (take 7#F as example, the gradient concentration were 1400, 700, 350, 175, 87.5 and 43.75).
Table 1: Investigational herb conditions design of Wuji Wan, L9(34) orthogonal table and Initial concentration.
| Isoenzyme | Substrate | Metabolites | IC50/μg(crude drug)•mL-1 |
|---|---|---|---|
| CYP1A2 | Phenacetin | Acetaminophen | <100 except WZY, BS, 2#F and 3#F, details in Table 3a (continue 1) |
| CYP2A6 | Coumarin | 7-hydroxy-coumarin | >100, details in Table 3b (continue 2) |
| CYP2C19 | Mephenetoin | 4-hydroxy-mephenetoin | <100 except WZY, BS,2#F and 3#F, details in Table 3c (continue 3) |
| CYP2D6 | Dextromethorphan | Dextrorphan | <100 except WZY, BS, 2#F and 3#F, details in Table 3d (continue 4) |
| CYP3E1 | chlorzoxazone | 6-hydroxy-chlorzoxazone | <100 except WZY, BS,2#F and 3#F, details in Table 3e (continue 5) |
| CYP3A1/3A2 | Testosterone | 6β-hydroxy-testosterone | <100 except WZY, BS,3#F , 4#F and 5#F, details in Table 3f (continue 6) |
Note: Inhibition of rat CYP1A2 and CYP3A1/3A2 by Wuji Wan were using the single enzyme/substrate method and for others 4 CYPs was using cocktail method.
Table 2: The IC50 values of Wuji Wan on the activities of six major CYP isoenzymes in rat liver microsomes..
The Table 1 shows that the ratios of 3 herbs combination in 9 different Wuji Wan Formulae, and the design inspiration of these ratios is from L9(34)orthogonal table. So we prepared the actual concentration of the 12 investigational herbs according to Table 1, these concentration also are shown in Table 1.
As showed in Table 3, single HL has strong inhibitory effects of 6 CYPs, while single WZY has a little bit inhibitory effects and single BS has no effect almost. if 3 herbs combination, the inhibiting effect of HL can be affected by the other 2 herbs. So the IC50 of HL for 9 Wuji Wan was took out from the whole Formula according the radio of HL within the Formula, the figure of single HL IC50 for 6 CYPs compare with the decomposition IC50 of HL in 9 Formulae as showed in Figure 1. the decomposition of WZY and BS IC50 in 1#~9#F were far lower than single herb, and shows extremely significant difference, it is mainly because 1#~9#F were added HL, the main material for the CYPs inhibitor.
| Wuji Wan Formulae | Decomposition IC50of Formula/μg(crude drug) •mL-1 | Formula IC50 | ||
|---|---|---|---|---|
| A: HL | B: WZY | C: BS | ||
| single HLextract | 28.07 ± 1.398 | - | - | 28.07 ± 1.398 |
| single WZY extract | - | 989.69 ± 110.953 | - | 989.69 ± 110.953 |
| single BS extract | - | - | 6633.28 ± 336.094 | 6633.28 ± 336.094 |
| 1#F extract | 24.82 ± 1.851 1) | 8.27 ± 0.617*) | 24.82 ± 1.851Ⅲ) | 57.92 ± 4.319 |
| 2#F extract | 28.47 ± 2.107 | 18.98 ± 1.405*) | 56.94 ± 4.214Ⅲ) | 104.38 ± 7.725 |
| 3#F extract | 45.90 ± 2.784 3) | 91.79 ± 5.568*) | 183.59 ± 11.136Ⅲ) | 321.28 ± 19.488 |
| 4#F extract | 14.85 ± 1.173 3) | 2.47 ± 0.195*) | 14.85 ± 1.173Ⅲ) | 32.17 ± 2.541 |
| 5#F extract | 24.03 ± 1.396 1) | 8.01 ± 0.465*) | 48.06 ± 2.791Ⅲ) | 80.09 ± 4.652 |
| 6#F extract | 28.59 ± 3.062 | 28.59 ± 3.062*) | 14.29 ± 1.531Ⅲ) | 71.47 ± 7.656 |
| 7#F extract | 36.84 ± 2.988 2) | 3.07 ± 0.249*) | 36.84 ± 2.988Ⅲ) | 76.76 ± 6.224 |
| 8#F extract | 28.53 ± 6.143 | 4.75 ± 1.024*) | 7.13 ± 1.536Ⅲ) | 40.41 ± 8.702 |
| 9#F extract | 14.72 ± 2.499 3) | 7.36 ± 1.249*) | 7.36 ± 1.249Ⅲ) | 29.45 ± 4.997 |
Note: (1) the decomposition IC50 was calculated by table 1 ratios;
(2) compared with the IC50 of HL, 1)P<0.05, 2)P<0.01, 3)P<0.001; compared with the IC50 of WZY, *) P<0.001; compared with the IC50 of BS,Ⅲ)P<0.001.
(3) Each data point represents the mean value (± SD) of two triplicate determinations, the same below.
Table 3a: The IC50 values of Wuji Wan on the activities of CYP1A2 ( 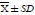 , n=6) (continue 1).
, n=6) (continue 1).
| Wuji Wan Formulae | Decomposition IC50of Formula/μg(crude drug) •mL-1 | Formula IC50 | ||
|---|---|---|---|---|
| A: HL | B: WZY | C: BS | ||
| single HLextract | 251.17 ± 33.90 | - | - | 251.17 ± 33.90 |
| single WZYextract | - | 1925.82 ± 241.66 | - | 1925.82 ± 241.66 |
| single BS extract | - | - | 2.18e+09 ± 5.3e+09 | 2.18e+09 ± 5.3e+09 |
| 1#F extract | 315.39 ± 41.06 1) | 105.13 ± 13.68*) | 315.39 ± 41.06Ⅲ) | 735.93 ± 95.82 |
| 2#F extract | 103.86 ± 3.47 3) | 69.24 ± 2.31*) | 207.73 ± 6.94Ⅲ) | 380.84 ± 12.74 |
| 3#F extract | 181.61 ± 9.38 2) | 363.23 ± 18.77*) | 726.46 ± 37.54Ⅲ) | 1271.31 ± 65.69 |
| 4#F extract | 135.44 ± 6.08 3) | 22.57 ± 1.01*) | 135.44 ± 6.08Ⅲ) | 293.46 ± 13.18 |
| 5#F extract | 147.21 ± 15.89 3) | 49.07 ± 5.29*) | 294.42 ± 31.78Ⅲ) | 490.71 ± 52.97 |
| 6#F extract | 113.32 ± 16.66 3) | 113.32 ± 16.66*) | 56.66 ± 8.33Ⅲ) | 283.30 ± 41.65 |
| 7#F extract | 286.09 ± 38.19 | 23.84 ± 3.18*) | 286.09 ± 38.19Ⅲ) | 596.02 ± 79.57 |
| 8#F extract | 161.38 ± 21.42 3) | 26.89 ± 3.57*) | 40.34 ± 5.35Ⅲ) | 228.63 ± 30.34 |
| 9#F extract | 211.33 ± 21.55 1) | 105.67 ± 10.78*) | 105.67 ± 10.78Ⅲ) | 422.68 ± 43.12 |
Note: (1) the decomposition IC50 was calculated by table 1 ratios;
(2) compared with the IC50 of HL,1)P<0.05, 2)P<0.01, 3)P<0.001; compared with the IC50 of WZY, *)P<0.001; compared with the IC50 of BS,Ⅲ)P<0.001.
Table 3b: The IC50 values of Wuji Wan on the activities of CYP2A6 ( 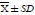 , n=6) (continue 2).
, n=6) (continue 2).
| Wuji Wan Formulae | Decomposition IC50of Formula/μg(crude drug) •mL-1 | Formula IC50 | ||
|---|---|---|---|---|
| A: HL | B: WZY | C: BS | ||
| single HLextract | 343.37 ± 69.09 | - | - | 343.37 ± 69.09 |
| single WZY extract | - | 1713.23 ± 464.29 | - | 1713.23 ± 464.29 |
| single BSextract | - | - | 20137.80 ± 14938.56 | 20137.80 ± 14938.56 |
| 1#F extract | 339.31 ± 35.32 | 113.10 ± 11.77*) | 339.31 ± 35.32Ⅲ) | 791.73 ± 82.42 |
| 2#F extract | 240.80 ± 15.92 1 | 160.53 ± 10.61*) | 481.61 ± 31.85Ⅲ) | 882.96 ± 58.40 |
| 3#F extract | 245.52 ± 32.93 1) | 491.04 ± 65.87*) | 982.09 ± 131.75Ⅲ) | 1718.66 ± 230.56 |
| 4#F extract | 136.59 ± 11.31 3) | 22.76 ± 1.88*) | 136.59 ± 11.31Ⅲ) | 295.95 ± 24.51 |
| 5#F extract | 192.45 ± 15.82 2) | 64.15 ± 5.27*) | 384.90 ± 31.65Ⅲ) | 641.50 ± 52.76 |
| 6#F extract | 277.41 ± 143.49 | 277.41 ± 143.49*) | 138.70 ± 71.74Ⅲ) | 693.54 ± 358.72 |
| 7#F extract | 174.95 ± 19.472) | 14.57 ± 1.62*) | 174.95 ± 19.47Ⅲ) | 364.49 ± 40.57 |
| 8#F extract | 128.18 ± 23.59 3) | 21.36 ± 3.93*) | 32.04 ± 5.89Ⅲ) | 181.59 ± 33.43 |
| 9#F extract | 133.19 ± 29.24 3) | 66.59 ± 14.62*) | 66.59 ± 14.62Ⅲ) | 266.39 ± 58.48 |
Note: (1) the decomposition IC50 was calculated by table 1 ratios;
(2) compared with the IC50 of HL,1P<0.05, 2)P<0.01, 3)P<0.001; compared with the IC50 of WZY, *)P<0.001; compared with the IC50 of BS,Ⅲ)P<0.001.
Table 3c: The IC50 values of Wuji Wan on the activities of CYP2C19 ( 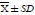 , n=6) (continue 3).
, n=6) (continue 3).
| Wuji Wan Formulae | Decomposition IC50of Formula/μg(crude drug) •mL-1 | Formula IC50 | ||
|---|---|---|---|---|
| A: HL | B: WZY | C: BS | ||
| single HLextract | 145.77 ± 35.24 | - | - | 145.77 ± 35.24 |
| single WZY extract | - | 1487.94 ± 254.04 | - | 1487.94 ± 254.04 |
| single BS extract | - | - | 2.84e+08 ± 6.52e+08 | 2.84e+08 ± 6.52e+08 |
| 1#F extract | 144.16 ± 16.59 | 48.05 ± 5.53*) | 144.16 ± 16.59Ⅲ) | 336.38 ± 38.72 |
| 2#F extract | 156.53 ± 1.72 | 104.35 ± 1.15*) | 313.06 ± 3.45Ⅲ) | 573.94 ± 6.34 |
| 3#F extract | 301.13 ± 18.31 3) | 602.27 ± 36.63*) | 1204.55 ± 73.26Ⅲ) | 2107.96 ± 128.20 |
| 4#F extract | 160.06 ± 27.98 | 26.67 ± 4.66*) | 160.06 ± 27.98Ⅲ) | 346.81 ± 60.62 |
| 5#F extract | 275.46 ± 2.78 3) | 91.82 ± 0.92*) | 550.93 ± 5.57Ⅲ) | 918.22 ± 9.29 |
| 6#F extract | 131.51 ± 24.14 | 131.51 ± 24.14*) | 65.75 ± 12.07Ⅲ) | 328.79 ± 60.37 |
| 7#F extract | 249.98 ± 14.47 3) | 20.83 ± 1.20*) | 249.98 ± 14.47Ⅲ) | 520.79 ± 30.15 |
| 8#F extract | 135.88 ± 18.31 | 22.64 ± 3.05*) | 33.97 ± 4.57Ⅲ) | 192.50 ± 25.94 |
| 9#F extract | 151.23 ± 69.57 | 75.61 ± 34.78*) | 75.61 ± 34.78Ⅲ) | 302.46 ± 139.14 |
Note: (1) the decomposition IC50 was calculated by table 1 ratios;
(2) compared with the IC50 of HL,1)P<0.05, 2)P<0.01, 3)P<0.001; compared with the IC50 of WZY, *)P<0.001; compared with the IC50 of BS, Ⅲ)P<0.001.
Table 3d: The IC50 values of Wuji Wan on the activities of CYP2D6 (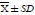 , n=6) (continue 4).
, n=6) (continue 4).
| Wuji Wan Formulae | Decomposition IC50of Formula /μg(crude drug) •mL-1 | Formula IC50 | ||
| A: HL | B: WZY | C: BS | ||
| single HLextract | 148.67 ± 39.02 | - | - | 148.67 ± 39.02 |
| single WZYextract | - | 1461.11 ± 170.40 | - | 1461.11 ± 170.40 |
| single BSextract | - | - | 8.02e+31 ± 1.96e+32 | 8.02e+31 ± 1.96e+32 |
| 1#F extract | 127.23 ± 10.76 | 42.41 ± 3.58*) | 127.23 ± 10.76Ⅲ) | 296.88 ± 25.12 |
| 2#F extract | 131.66 ± 2.94 | 87.77 ± 1.96*) | 263.33 ± 5.89Ⅲ) | 482.78 ± 10.81 |
| 3#F extract | 221.22 ± 14.55 2) | 442.45 ± 29.09*) | 884.91 ± 58.19Ⅲ) | 1548.60 ± 101.85 |
| 4#F extract | 95.51 ± 17.23 1) | 15.91 ± 2.87*) | 95.51 ± 17.23Ⅲ) | 206.94 ± 37.34 |
| 5#F extract | 177.27 ± 3.98 | 59.09 ± 1.32*) | 354.54 ± 7.97Ⅲ) | 590.91 ± 13.29 |
| 6#F extract | 79.50 ± 14.96 2) | 79.50 ± 14.96*) | 39.75 ± 7.48Ⅲ) | 198.75 ± 37.41 |
| 7#F extract | 155.20 ± 7.96 | 12.93 ± 0.66*) | 155.20 ± 7.96Ⅲ) | 323.35 ± 16.59 |
| 8#F extract | 101.69 ± 11.64 1) | 16.94 ± 1.94*) | 25.42 ± 2.91Ⅲ) | 144.06 ± 16.50 |
| 9#F extract | 91.47 ± 43.93 1) | 45.73 ± 21.96*) | 45.73 ± 21.96Ⅲ) | 182.94 ± 87.87 |
Note: (1) the decomposition IC50 was calculated by table 1 ratios;
(2) compared with the IC50 of HL,1)P<0.05, 2)P<0.01, 3)P<0.001; compared with the IC50 of WZY, *)P<0.001; compared with the IC50 of BS, Ⅲ)P<0.001.
Table 3e: The IC50 values of Wuji Wan on the activities of CYP2E1 ( 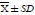 , n=6) (continue 5).
, n=6) (continue 5).
| Wuji Wan Formulae | Decomposition IC50of Formula/μg(crude drug) •mL-1 | Formula IC50 | ||
|---|---|---|---|---|
| A: HL | B: WZY | C: BS | ||
| single HLextract | 38.96 ± 3.377 | - | - | 38.96 ± 3.377 |
| single WZYextract | - | 871.96 ± 59.277 | - | 871.96 ± 59.277 |
| single BSextract | - | - | 15519.17 ± 1438.261 | 15519.17 ± 1438.261 |
| 1#F extract | 18.50 ± 1.148 3) | 6.17 ± 0.383*) | 18.50 ± 1.148Ⅲ) | 43.17 ± 2.678 |
| 2#F extract | 16.49 ± 1.335 3) | 11.00 ± 0.890*) | 32.99 ± 2.669Ⅲ) | 60.47 ± 4.893 |
| 3#F extract | 39.45 ± 4.460 | 78.89 ± 8.919*) | 157.78 ± 17.838Ⅲ) | 276.12 ± 31.217 |
| 4#F extract | 61.57 ± 3.724 3) | 10.26 ± 0.621*) | 61.57 ± 3.724Ⅲ) | 133.40 ± 8.068 |
| 5#F extract | 35.42 ± 2.469 | 11.81 ± 0.823*) | 70.85 ± 4.937Ⅲ) | 118.08 ± 8.229 |
| 6#F extract | 35.39 ± 1.374 1) | 35.39 ± 1.374*) | 17.69 ± 0.687Ⅲ) | 88.47 ± 3.434 |
| 7#F extract | 30.89 ± 2.434 2) | 2.57 ± 0.203*) | 30.89 ± 2.434Ⅲ) | 64.36 ± 5.072 |
| 8#F extract | 24.80 ± 1.118 3) | 4.13 ± 0.186*) | 6.20 ± 0.280Ⅲ) | 35.13 ± 1.584 |
| 9#F extract | 19.95 ± 2.345 3) | 9.98 ± 1.172*) | 9.98 ± 1.172Ⅲ) | 39.91 ± 4.690 |
Note: (1) the decomposition IC50 was calculated by table 1 ratios;
(2) compared with the IC50 of HL,1)P<0.05, 2)P<0.01, 3)P<0.001; compared with the IC50 of WZY, *)P<0.001; compared with the IC50 of BS, Ⅲ)P<0.001.
Table 3f: The IC50 values of Wuji Wan on the activities of CYP3A1/3A2 ( 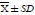 , n=6) (continue 6).
, n=6) (continue 6).
As showed in Figure 1, compared with single HL, the decomposition inhibition of HL for 6 CYPs in 9 Wuji Wan Formulae have the different effects, some have more strong inhibition than single HL, another hand, some have less inhibitory effects. So we further analysis the relationship of decomposition inhibition of HL for 6 CYPs by SPSS11.5 with one-way analysis of variance (ANOVA) and multi-factor analysis of variance. Due to the different experimental methods and the deviation of the test result, there are two CYPs among the 6 CYPs, CYP1A2 and CYP3A1/3A2, have statistical significance of decomposition IC50 of HL analyzed by ANOVA and multi-factor analysis of variance, the results as showed below:
The results of ANOVA
Between the CYP1A2 and CYP3A1/3A2 test, Pairwise comparison among group of Formulae 1-9#, the decomposition IC50 of HL were significantly different, it’s showed that the different compatibility radio of three herbs in Wuji Wan 1-9# Formulae can cause different inhibitory effects to CYP1A2 or CYP3A1/3A2.
The results of multi-factor analysis of variance
CYP1A2 [12,20] Among 1-9# Formula, according to L9(34) orthogonal table design, there are 3 dose levels of HL, WZY and BS, the inhibitory test results show that HL and 1~9# of Wuji Wan can inhibit the enzymic activity of CYP1A2 significantly, and the capability of HL in Wuji Wan of action on CYP1A2 can be modified by different composition of WZY and BS in Wuji Wan, and there are statistical difference among the decomposition IC50 of 1~9# of Wuji Wan; while the ratio of HL and WZY raising up in Wuji Wan, Wuji Wan may supperess the enzymic activity of CYP1A2 strengthenly and with the ratio of BS raising up in Wuji Wan, the suppressed capability of Wuji Wan on CYP1A2 should be weaken oppositely.
The IC50 of Rhizoma Coptidis(HL), Fructus Evodiae Rutaecarpae(WZY), Radix Paeoniae Alba(BS) and Formula 1~9# are: 28.07, 989.69, 6633.28, 57.92, 104.38, 321.28, 32.17, 80.09, 71.47, 76.76, 40.41 and 29.45 μg(crude drug)/mL, respectively.
CYP3A1/3A2 [34] Among 1-9# Formula, there are 3 dose levels of HL, WZY and BS, the inhibitory test results show that HL and 1~9# of Wuji Wan can suppress the enzymic activity of CYP3A1/3A2 significantly, and the capability of HL in Wuji Wan of action on CYP3A1/3A2 can be modified by different composition of WZY and BS in Wuji Wan, and there are statistical difference among the decomposition IC50 of 1~9# of Wuji Wan; while the ratio of HL raising up in Wuji Wan, Wuji Wan may supperess the enzymic of CYP3A1/3A2 strengthenly and with the ratio of BS raising up in Wuji Wan, the suppressed capability of Wuji Wan on CYP1A2 should be weaken oppositely.
The IC50 of HL, WZY, BS and Formula 1~9# are: 38.96, 871.96, 15519.17, 43.17, 60.47, 276.12, 133.40, 118.08, 88.47, 64.36, 35.13 and 39.91 μg (crude drug)·mL-1, respectively.
Materials
HL, WZY and BS were purchased from Wei Ren crude herbal drug factory (Beijing, China). HL ethanol extract, WZY ethanol extract and BS water and ethanol mixture extracts were prepared by China-Japan Friendship Hospital as previously described [12], extracts yield were 23.75%, 28.33% and 4%, respectively. Different Wuji Wan Formulae were prepared as described in Table 1.
Phenacetin, Coumarin, Mephenetoin, Dextromethorphan, chlorzoxazone, Testosterone and theirs metabolites acetaminophen, 7-hydroxy-coumarin, 4-hydroxy-mephenetoin, Dextrorphan, 6-hydroxy-chlorzoxazone, 6β-hydroxy-testosterone were purchased from Sigma (St. Louis, MO, USA). 6-glucose phosphate, β-naphthoflavone also were purchased from sigma company; Other reagents includes the oxidized coenzyme II (Beijing zhongheng Ltd.), phenobarbital sodium injection (Specification: 0.1 g/mL; Tianjin Amino Acid Ltd.), BCA kit (Beijing Seitz biological Technology Ltd.), precision pH paper, acetonitrile (HPLC grade, Tedia, U.S.), methanol (HPLC grade, Tianjin siyou reagent company), purified water, magnesium chloride (analytical grade), potassium chloride (analytical grade), potassium hydroxide (analytical grade) and phosphoric acid.
Male Wistar rats (200 ± 20 g) were used and supplied by Beijing Vital Laboratory Animal Technology (Beijing, China) and housed under standard conditions of temperature, humidity and light, and had free access to standard rodent diet and water before the experiment. The animal protocol was approved by the Animal Ethics Committee at the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
Instrument
HPLC chromatograph (Waters 2695 autosampler, Waters 2489- UV detector, Empower Data Processing System, U.S.), a Kromasil-C18 column (250 mm × 4.6 mm, 5 μm), Sorvall super T21 high-speed centrifuge, Japan MDF-U 50 V ultra-cold freezer (-80°C); continuous sample injector; BHW-2 thermostatic water bath tank; analytical balance; homogenizer.
The LC–MS/MS method was performed using a Waters Xevo TQ MS System (Waters, Milford, MA, USA). The system was controlled with MassLynx V4.1 software (Waters, Miford, MA, USA) for data acquisition and analysis. The LC separation was carried out on a HPLC Clipeus-C8 column (30 mm×2.1 mm, 5 μm), mobile phase: mobile phase A: Water; mobile phase B: methanol; mobile phase C: 5 mM ammonium acetate.
Preparation of rat liver microsomes
Liver microsomes were prepared from male Wistar rats according to the Reference method [35,36]. In brief, rats were induced by β-naphthoflavone and phenobarbital sodium, then the liver microsomes were made with high-speed centrifugal method: 1st day each rat intraperitoneal injection of 30 mg • kg-1 dose of phenobarbital sodium, 2nd to 4th day, injection of 60 mg • kg-1 dose of phenobarbital sodium, the same time, 3rd to 4th day, intraperitoneal injection dose of 80 mg • kg-1 β-naphthoflavone; 4th night, The rats were fasted for 12 hours, the next day rats were sacrificed by femoral artery cutting and then: open the abdominal cavity and chest cavity, separate hepatic artery and portal vein, rinse the liver by Portal vein perfusion with cold potassium chloride (0.15 mol · L-1) buffer (pH7.4), cut the hepatic artery for KCl buffer excretion after the liver was rinsed expansion, repeat portal vein perfusion till the color of liver became khaki; remove liver and cut into pieces in petri dish, and then: per gram of liver tissue added 3 mL cold potassium chloride (0.15 mol • L-1) buffer, homogenate was prepared in an ice bath; collected homogenate 9000 g centrifugal 15 min for the first time, took the supernatant for 105000 g ultracentrifugation 60 min, the second supernatant was discarded, and the precipitate was resuspended in 0.15 mol • L-1 ice-potassium chloride buffer, again 105000 g ultracentrifugation 60 min, the precipitate was resuspended (i.e. rat liver microsomes) for packaging per gram 4 mL 0.15 mol • L-1 ice KCl buffer, -80°C stored. With solcoseryl albumin as standard substance, the microsomal protein content was measured using BCA kit method, the protein concentration was 1.2 g • L-1.
The Liver mixed microsomal Enzymes solution preparation method
Drugs affect CYP450 isoenzyme activity in vitro: for the determination of drug IC50 values of the 6 CYPs isoenzymes in this study, 6 drug concentrations and a solvent control, total 7 test conditions were seted, per 1 condition parallel three incubation tubes, an investigational drug each time to be done 21 incubation tubes, each time need the preparation of 12 mL microsomal enzymes solution: the liver microsomal suspension 1.2 mL as "3.3" item, 0.4 mol • L-1 Mgcl2 solution 120 μL, 1.65 mol • L-1 Kcl solution 120 μL,6 - glucose phosphate 15 mg, the oxidized coenzyme II 20 mg, pure water 4.56 mL, 0.2 mol•L-1 phosphate buffer solution (pH 7.4) 6 mL, shocking mixed; The mixed enzyme solutions were made temporarily for fresh activity in an ice bath.
Above method just for one investigational drug experiment, if there are 2 or more drugs experiments at the same time, it is better for just set one solvent control to save cost for reducing the experimental mixed enzymes solution.
CYP1A2, CYP3A1/3A2, CYP2A6, CYP2C19, CYP2D6 and CYP2E1 enzyme metabolic activity detection
CYP1A2 activity was measured by incubation experiments in vitro: firstly, draw120 μL concentration of 100 μmol • L-1 mark metabolic substrate of CYP1A2 - phenacetin solution added to the "3.4" item 12 mL liver microsomal enzyme system solution, and mix evenly, then added to 21 incubation tubes, 0.5 mL per incubation tube, also added 5 μL investigational drug solution per incubation tube, mixing. The above operation carried out in an ice bath. Finished adding the investigational drug, the substrate of CYP1A2 and the liver microsomal enzymes, the incubated tubes were set at 37°C water bath for 3 h, then the reaction was terminated by adding 0.5 mL cold methanol, transferred to 1.5 mL bullet centrifuge tube, 4°C refrigerator placement 3 h for protein precipitation, then 18000 r•min-1 centrifugal 20 min, took the supernatant 20 μL, HPLC injection for determination of phenacetin metabolites - acetaminophen, the concentration of acetaminophen and CYP1A2 enzyme activity was positively related. CYP3A1/3A2, CYP2A6, CYP2C19, CYP2D6 and CYP2E1 enzyme metabolic activity detection are the same as CYP1A2, just take corresponding enzyme/ substrate as Table 2.
HPLC detection method
5.6.1 CYP1A2 test HPLC method: Kromasil-C18 column (250 mm × 4.6 mm, 5 μm); mobile phase A: 5% acetonitrile, 15% methanol and 80% water; mobile phase B: 10% acetonitrile, 60% methanol and 30% water; gradient elution procedure. : 0 min (100% A), 8 min (80% A, 20% B), 12 min (60% A, 40% B), 20 min (100% A); run time: 40 min; flow rate: 1 mL • min-1; Column temperature: 30 °C; detection wavelength: 245 nm; injection volume: 20 μL.
CYP3A1/3A2 test HPLC method [27]: Kromasil-C18 column (250 mm × 4.6 mm, 5 μm); mobile phase A: 10% acetonitrile, 60% methanol,30% water; mobile phase B: water; mobile phase C: acetonitrile; gradient elution program: 0 min (65% A, 35% B), 4.5 min (65% A, 35% C), 8 min (65% A, 35% B); running time: 13 min; flow rate: 1 mL•min-1; column temperature: 30°C; detection wavelength: 245 nm; injection volume: 20 μL.
CYP2A6, CYP2C19, CYP2D6 and CYP2E1 cocktail test LC-MS method [3]: Clipeus-C8 column (30 mm×2.1 mm, 5 μm), the gradient program was as follows: 0 min (90% A, 10% B), 1.0 min (90% A, 10% B), 2.0 min (60% A, 40% B), 3.5 min (60% A, 40% B), 3.51 min (40% B, 60% C), 4.5 min (60% B, 40% C), 4.51 min (40% A, 60% B), 5.0 min (20% A, 80% B), 6.0 min (20% A, 80% B), 6.01 min (90% A, 10% B), 8.0 min (90% A, 10% B); running time: 8 min; flow rate: 0.2 mL•min-1; column temperature: 25°C. High purity nitrogen served as both nebulizing and drying gas. Drying Gas Flow: 10 L•min-1; Drying Gas Tem: 350°C; MS: ESI ion Source, positive and negative mode while testing, MRM scan mode: 7-hydroxycoumarin, 163-189 positive ions; 4-hydroxy Meifen properly due, 201-230 negative ions; the right of non-methane, 269- 233 positive ions; 6 - hydroxy-chlorzoxazone 184-184 negative ions.
Methodology coefficient of recovery, the minimum detection limit, Analysis method stability and precision determination All of these items can fulfil the experimental requiring.
HPLC and LC-MS chromatogram: Figures 2-4.
Experimental data statistical method
The inhibition ratio of different concentrations investigational drug on CYPs activity: inhibition ratio (%)= (probe metabolite generation amount without investigational drug - metabolite generation amount with investigational drug) / metabolite generation amount without investigational drug × 100%; then calculate the IC50 values with reforming Bliss method. Data expressed as X ± SD and using SPSS 11.5 to calculate t test, one-way ANOVA and multivariate analysis of variance [13-15].
In conclusion, the present study demonstrated that Wuji wan showed significant inhibitory effects on CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A1/3A2; while 3 herbs were used singly, Rhizoma Coptidis (HL) has strong inhibitory effects of 6 CYPs, Fructus Evodiae Rutaecarpae (WZY) has a little bit inhibitory effects and Radix Paeoniae Alba (BS) has no effect almost. Moreover, Wuji Wan 1-9# Formulae developed from orthogonal table L9(34) showed different inhibitory effects of 6 CYPs (statistical difference, Pï¼?0.05 or 0.01), some Formulae have more strong inhibition than single HL, some Formulae have the same inhibitory intension as HL approximately and some Formulae have less inhibitory effects than HL; Namely, if 3 herbs combination, the inhibiting effect of HL(the main material for the CYPs inhibitor) can be affected by the other 2 herbs, it’s depend on the combination ratio of 3 herbs in Wuji Wan. Further analysis showed that there are 3 dose levels of HL, WZY and BS in Wuji Wan Formulae, while the ratio of HL and/or WZY raising up in Wuji Wan, Wuji Wan may inhibit the enzymic activity of CYPs strongly and with the ratio of BS raising up in Wuji Wan, the inhibitory capability of Wuji Wan of CYPs should be weaken oppositely; These Features showed the statistical significance in some CYPs test, for example, in CYP1A2 and CYP3A1/3A2 test.
So, the reason why Wuji Wan with different combination has different pharmacodynamics and pharmacokinetics features is likely to lie in the difference of the inhibitory capability on CYP450 of Wuji Wan with different proportion, because when a patient be offered Wuji Wan with different combination, Wuji Wan can change the enzymic activity of CYP450 then make the patient has the different Wuji Wan prototype/metabolites features, thereby, producing different therapeutic effects.
Also, it’s should be noticed that Rhizoma Coptidis or Wuji Wan is likely to cause clinically significant herb-drug or herb-herb interactions and thus cause the occurrence of adverse drug reactions in humans when co-adminstrated with subtrates of the 6 CYPs [37].
This study was supported by Beijing Natural Science Foundation (7152105), National Natural Science Foundation of China (30930114), Sino-Austria international cooperation project (No.2014DFG32700) and Autonomous Project of China Academy of Chinese Medical Sciences (ZZ0708 and ZZ03060).