Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2017) Volume 8, Issue 3
Keywords: Heat inactivation; Peroxidase; Solanum ethiopicum
Peroxidase (1. 11. 1. 7), an important enzyme that catalyzes the oxidation of a large variety of phenolic substrates through the reaction with hydrogen peroxide [1]. They represent a large family of heme containing enzymes [2] that are widely distributed in microbes, plants and animals [3]. Different researchers have reported the extraction of peroxidase from various plant sources such as Carica papaya , pears and apples [4-6].
Peroxidases are versatile biocatalysts with wide number of applications [7]. They have been employed in the removal of endocrine disruptive chemicals [8], diagnostic kits [9] and in pharmacological aspects [10].
Solanum ethiopicum is a good source of peroxidase [11]. We consider the parameters needed to characterize the thermal stability of peroxidase from this source to serve as a possible alternative to the classical horseradish peroxidase for industrial purposes. Such parameters as outlined by [12] include the D-value. Z-value and Ea. Also, the temperature dependence of the reaction rates could be derived from Arrhenius’ law [13]. This research reports the energetics for the thermal inactivation of peroxidase from green colored S. ethiopicum (green) fruit.
Solanum ethiopicum fruit was harvested from a farm in Edem-ani, Nsukka L. G. A. of Enugu state. O-dianisidine was product of Sigma (England). All other chemicals and reagents were of analytical grade.
Extraction of peroxidase
Extraction of peroxidase was carried out according to the method of [12]. Protein content was estimated according to the method of [14].
Enzyme assay
Peroxidase activity was assayed using the method of [15] by measuring the change in absorbance as it oxidizes the o-dianisidine on addition of hydrogen peroxide. The assay mixture contained 2 ml of 100 mM phosphate buffer (pH 6.0), 0.3 ml of substrate (o-dianisidine), and 0.4 ml of crude enzyme. The reaction was initiated by adding 0.2 ml of 0.8% hydrogen peroxide. The peroxidase activity was monitored by change in absorbance (470 nm) due to the oxidation of odianisidine using Jenway 6305 UV/VIS spectrophotometer.
Therma treatment
Kinetic parameters were estimated as described by [15]. The kinetics of thermal inactivation of peroxidase from the fruit of Solanum ethiopicum were determined based on inactivation experiments for a period of 90 min in a temperature controlled water bath (Gallenkamp, England). The enzyme solution was placed in a pre-warmed tube at temperatures of 30, 40, 50, 60 and 70°C, and aliquots were withdrawn using a micropipette at 30 min time intervals. Afterwards, the samples were immediately cooled in ice water to stop the thermal inactivation process. The residual enzyme activity was then measured as described in the peroxidase assay section. The stability of the enzyme was expressed as percentage residual enzyme activity.
Calculations
Energetic calculations and analysis were carried out as outlined by [16], using data derived from energetic indices of the enzyme.

A specialized parameter used in the characterization of enzyme stability is the decimal reduction time (D-value), which is the time required for one log10 reduction in the concentration or activity of the reacting species [16]. It is calculated from using the first order constant (kd) in a straight forward pattern
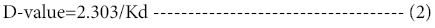
Similarly, Z-value is the temperature increase required for a onelog10 reduction in the D-value. It can be calculated from a plot of log 10 D versus temperature
The change in enthalpy, entropy and free energy of denaturation was calculated directly from the following equations
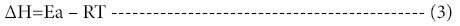
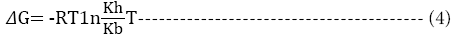
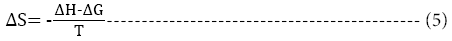
Ea is the activation energy of inactivation; T is the absolute temperature (K); R is the gas constant (8.314 Jmol-1K-1), kh is the plancks constant (11.04 × 10-36 Jmin-1), kb=Boltzmann constant (1.38 × 10-23 JK-1).
The kinetics of thermal inactivation of peroxidase extracted from the fruit of Solanium ethiopicum was monitored at the temperature range of 30-70°C using suitable kinetics parameters. The percentage residual activity (Figure 1) and Ln percentage residual activity of the enzyme (Figure 2). The Z–value was calculated from Figure 3.
Table 1 and Figure 4 show the various thermodynamic parameters used in characterizing the enzyme. It was observed that the t1/2 was decreased as the temperature increased from 30-50°C. At 60°C, the t1/2 increased to 78 min, showing that the time taken for loss of 50% of the enzyme activity was higher than that obtained for 50°C as shown in Table 1. Also, denaturation constant (Kd) were 0.0066, 0.0077, 0.0098, 0.0088 and 0.0166 respectively for the temperatures studied. Similarly, the time required to reduce the peroxidase activity by 90% (D-values) were 383.33, 294.87, 234.69, 261.36 and 198.27 for 303, 313, 323, 333 and 343K respectively. It was observed that the D-values were increasing as the time of incubation increased, this implies prolonged enzyme inactivation at higher temperature [12].
| T (°K) | Kd | t1/2(min) | D-value | ΔH(KJ/mol) | Ea (KJ/mol) | ΔG(KJ/mol) | ΔS(KJ/mol) |
|---|---|---|---|---|---|---|---|
| 30 (303) | 0.0060 | 115.5 | 383.33 | -3514.06 | -994.92 | 1.64 | -11.6 |
| 40 (313) | 0.0078 | 88.84 | 294.87 | -3596.86 | -994.58 | 6.92 | -11.51 |
| 50 (323) | 0.0098 | 70.71 | 234.69 | -3679.72 | -994.30 | 15.13 | -11.43 |
| 60 (333) | 0.0088 | 78.75 | 261.36 | -3762.79 | -994.23 | 9.86 | -11.32 |
| 70 (343) | 0.0116 | 59.74 | 198.27 | -3845.86 | -994.16 | 20.67 | -11.27 |
| Z-Value=0.0409 | |||||||
Table 1: Half-life and activation parameters for the thermal inactivation of PURE HNGE peroxidase.
The change in entropy (ΔS°) obtained were -11.60, -11.51, -11.43, -11.32 and -11. 27 KJmol-1 respectively for temperatures studied. A negative ΔS° value shows a decrease in the disorder or randomness of the protein during denaturation [16]. This infers that the peroxidase is stable, as the rate of randomness of the enzyme was low within the temperature range studied.
On the other hand, the change in Gibb’s free energy values of the enzyme were 1.64, 6.92, 15.13, 9.86 and 20.67 K Jmol-1 for the respective temperatures studied. It was observed that change in free energy increased with the temperatures of incubation. The positive values of free energy obtained from the study suggests that the process is less spontaneous as more energy is required for the process to occur. [16], reported that a more negative ΔGo value is associated with a more spontaneous process. Enzyme molecule with high ΔGo is considered to be stable [15]. The data obtained here were higher than the ΔGo values of -87391 kJ/mol observed for oil bean seed peroxidase [15].
The activation energy of denaturation (Ea) calculated for the temperatures were -994.92, -994.58, -994.30, -994.23 and -994.16 KJmol-1 respectively. The values increased with increase in temperature. Activation energy of 67.67 kJmol-1 was obtained for edible yam peroxidase. Similarly, activation energy of -2.983 kcal/mol was revealed for sorghum peroxidase. Marangoni [16] stated that higher value of Ea is indicative of a thermostable enzyme as more energy is required for enzyme denaturation to occur. The activation energy (Ea) for peach fruit peroxidase was reported as 7.97 Kcal/mol. Activation energy of potato peroxidase was obtained as 27.114 KJ/mol [1]. High Ea value shows that the process is strongly temperature dependent, at lower temperature, this rate becomes insigni icant [12]. The results of this study imply that high temperature and short time could aid in extending the shelf life of the fruit.
The findings of the thermodynamic indices (change in enthalpy, entropy and free energy) suggests that peroxidase extracted from the fruit of S. ethiopicum is thermally stable. The high Ea values obtained from the study could be attributed to thermostability of the enzyme as more energy would be required to denature the enzyme. Also, the positive values for the free energy and negative changes in the entropy indicate that the denaturation was less spontaneous and more orderly respectively.