Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2010) Volume 1, Issue 1
The effect of heat treatment on the activities of three quality related enzymes:- peroxidase (POD), polyphenol oxidase (PPO), and lipoxygenase (LOX), from edible white yam (Dioscorea rotundata) was studied over a temperature range of 50 to 80°C using mathematical analysis of the kinetic and thermodynamic parameters for the thermoinactivation of the enzymes. Denaturation of these enzymes, measured by loss in activity, could be described by a simple first-order reaction that was resolved into biphasic inactivation curves. This indicates the existence of two isoforms of different thermal stabilities with k-values between 0.032 and 0.525. D-values decreased with increasing temperature, indicating faster inactivation of the enzymes at higher temperatures. Results suggested that peroxidase is relatively more thermostable than polyphenol oxidase and lipoxygenase with a Z-value of 4.11, Ea of 2510kJ mol-1 for the first phase of the biphasic inactivation reaction. The Gibbs free energy (ΔG), values range from -552.95 to 279.01kJ/mol for the three enzymes. The results indicate that the oxidation reactions were: (1) not spontaneous (ΔG > 0) for peroxidase at low temperatures, (2) spontaneous (ΔG < 0) for lipoxygenase (3) slightly endothermic (ΔH > 0) for lipoxygenase and (4) reversible (ΔS < 0) for all the three enzymes at all temperature. The high z-value obtained for peroxidase in the first phase of inactivation indicates that a high amount of energy was required to initiate its denaturation, and supports why it is used as a marker for inactivation of quality-related enzymes.
Keywords: Enzyme activity; Thermo-inactivation; Half-life; Kinetics; Thermodynamics; Quality-related enzymes
It has been widely reported that the presence of residual endogenous enzymes in both raw and processed fruit and vegetable products may cause loss of quality during storage [1,2].
Yam is an important tropical crop [3], particularly in the form of processed products. Of these, yam flour, derived from crushing and grinding the boiled yam tuber is marketed at a premium price as a high value added product. Important to the preparation of this is the inactivation of some quality related enzymes involved in the deterioration of the finished products. Post harvest deterioration of white yam tuber has made it difficult to obtain yam products with their original white color desired by consumers [4,5], even though the first step in the processing involves boiling of the tubers. Since such heat treatments could lead to loss of desirable characteristics, it is important to keep the heat treatments at a minimum, while being sufficient to completely inactivate the deleterious enzymes.
Peroxidase (POD), lipoxygenase, (LOX), and polyphenol oxidase, (PPO), have been chosen for this study because they are involved in the deterioration of food products during harvest, storage, and processing [6,7,8,9]. POD, an indicator enzyme with high thermal stability, is involved in off-flavour developments, but an attempt to completely inactivate it may lead to other quality related problems. It has also been, observed that those enzymatic reactions catalyzed by LOX, results in the development of an undesired aroma [6,7]. Dicko et al. [10], observed that horticultural products suffer both quantitative and qualitative losses due to the oxidation of the phenolic compounds or the undesired enzymatic activities of enzymes such as PPO which decrease the market value of these products. Thermal inactivation profiles of these enzymes follows first order kinetics with the time required varying with the product of the studies. On heat inactivation of these quality related enzymes only a few studies on heat inactivation of these quality-related enzymes have included the calculations of Arrhenius and the kinetic parameters from various foods [11,12,13]. To be able to optimize heat treatments of white yam in order to maximize quality, a model is developed for inactivation kinetics for these enzymes in order to be able to predict quality changes during processing and subsequent storage.
We consider the parameters needed to characterize the thermal stability of a given enzyme: the D-value (the duration of heat treatment at a given temperature required to reduce activity by 10 % of its original value), and Z-value (also known as the thermal destruction time), is the temperature increase [in Kelvin’s] required to reduce D by 90 %), also the Ea, determines the rate of enzyme inactivation at any temperature with the expected level of residual activity after a given heat treatment which help to develop a model. For the yam chosen for this study, the existing data on thermal inactivation of the enzymes are inadequate with reference to thermodynamic studies [14,15].
We report here more detailed analysis of the kinetics and thermodynamic parameters for the thermal inactivation of three quality related enzymes and a model for such process in white yam.
Preparation of homogenates
Fresh uninfected yam tubers were peeled, washed and diced into ≈1cm cubes. Fifty grams of the cubes were soaked in 50ml of ice – cold 0.025M sodium phosphate buffer pH 7.5 and homogenized in a warring blender. The homogenate was filtered through four layers of cheese cloth and centrifuged at 10,000xg for 20min in refrigerated centrifuge. The supernatant was desalted by passage through sephadex G 25 equilibrated with 0.01M phosphate buffer pH 7.5, to remove phenolic compounds that can cause rapid browning in the cubes. All solutions used in the preparation of the homogenates as well as the plant materials were kept on ice.
Thermal inactivation
The enzyme was exposed to different temperatures and at suitable interval of time. The method used here was that of McLellan and Robinson [16]. The homogenates were kept on ice until heating.
Samples were heated in a circulating water bath at 50, 60, 70, and 80°C respectively. At suitable intervals of time, 0.1ml of the homogenate was withdrawn for activity determination. To minimize lag phase, 4ml of phosphate buffer pH 7.5 were pre-incubated to the required temperature and 1ml of the homogenate was added and rapidly mixed. Following heating, samples were cooled on cold water and stored on ice until assay.
Enzyme assays
Peroxidase activity was assayed using the method of McLellan and Robinson [16]. The change in absorbance at 460 nm due to the oxidation of o-dianisidine in the presence of H2O2 and enzyme at 30°C was monitored using a Pye-Unicam SP 460 spectrophotometer (Pye-Unicam Ltd, Cambridge UK). The standard assay solution contains 0.3ml of o-dianisidine, 2.6 ml of 0.1 M sodium phosphate buffer pH 6.0, and 0.1 ml of enzyme extract, in a total volume of 3.0 ml. One unit of enzyme activity was defined as the amount of enzyme that gives an absorbance change of 0.1/min at 30°C.
Assay method for polyphenol oxidase activity was that of Chutintrasri and Noomhorm [12]. One unit of PPO activity is defined as a change in absorbance of 0.001/min at 400 nm using 3.3 mM catechol solution prepared in 0.1M sodium phosphate buffer (pH 7.0).
Lipoxygenase assay was according to the method of Anthon and Barrett, [1]. The reaction was started by addition of 0.1 ml of LOX (5times dilution with water with pH adjusted to 7.0 with HCl) and the increase in absorbance at 234 nm was read every 30 sec. One unit of enzyme activity is defined as the change in absorbance of 0.01/min under the conditions of assay.
Calculations
The rate constants k1 and k2 for the first and second phase of inactivation were calculated from linear regression of experimental enzyme retention data of the slopes of log % residual activity against time. The rate constants were re-plotted in Arrhenius plots, and the activation energies, Ea, were calculated using the Arrhenius equation:
Where R is the gas constant (8.314 mol-1K-1) and T is the temperature in K. Slopes and their standard errors were calculated by linear regression. The natural logarithms of K-values (ln K) for each time-temperature treatment were plotted vs. the reciprocal of heating temperatures (1/T). The Ea values were computed by multiplying the slope of the best fit line by the gas constant (1.987 cal mol-1deg-1(K).
The changes in enthalpy (ΔH), entropy (ΔS) and free energy (ΔG) of the enzyme inactivation were determined using equation 2, 3, and 4.
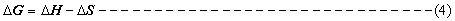
Where KB = Boltzmann constant (1.3807x10-23J/K), hp = Planks constant (6.62607x10-34m2kg/s), R = gas constant (8.314Jmol-1K-1), T is temperature in Kelvin and A is the Arrhenius constant.
D-value, was calculated by using
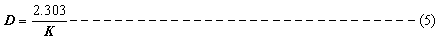
The half-life, t1/2 i.e. the time required for 50% reduction of initial activity at any given temperature was determined with equation (6)
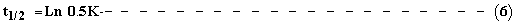
All the equations used here [1-6] were those of Anthon and Barret [1].
The rate of thermal inactivation of three quality-related enzymes in white yam was measured over the temperature range 50°C-80°C. It was observed that the time and temperature of the heating process affected the rate of inactivation of the enzyme. The residual enzyme activities for peroxidase (POD), Lipoxygenase (LOX), and Polyphenol oxidase (PPO), in white yam, are shown in figs 1a-c, respectively. The half-life of inactivation (t1/2), at each temperature was calculated from equation 6, above. All the three enzymes showed a steep decrease in t1/2 with increasing temperature. The t1/2-values calculated from k2 were also higher than the values obtained for k1 for the three enzymes studied. In white yam, about half of the POD activity was rapidly inactivated after 2.05, 2.86, 3.40 and 3.79 min of incubation at 80, 70, 60°C and 50°C, respectively. Similar observations were made for LOX and PPO. The time required for half the original enzyme activity (t1/2 values) for the rapid phase for LOX were 1.34, 1.67, 2.49 and 4.05 min at 80, 70, 60 and 50°C, respectively. This rapid loss of activity is assumed to be due to the inactivation of the labile isoform of the enzyme. The remaining half of the enzyme activities were inactivated more slowly. This second slow phase might arise from the inactivation of the more resistant form. Labile and resistant forms of POD are known to exist in guava [17]. Sergio et al.,[18], and Shalini et al., [19], noted that in plant crude extracts, the non-linearity of heat inactivation of peroxidase may be due to the presence of a number of isoperoxidases with different thermostability. Inactivation of the three quality-related enzymes for a few minutes of heating at selected temperatures of 50, 60, 70, and 80°C, shows non-linear kinetics. Nonlinear heat inactivation curves for POD, may occur because there are two steps involved, that is dissociation of haem from the active enzyme and a conformational change in the apoenzyme [20].
Figures 1a-c shows that inactivation curves for POD, LOX and PPO at 50- 80°C were biphasic. Biphasic curves were observed for the thermal inactivation of PPO and POD from yam [14]; POD from sorghum [16]; POD and PPO from Banana puree [21]; and LOX from walnuts and almonds [22]. Biphasic inactivation curves could be a multistep process involving stable intermediates [2], or involves the formation of thermostable aggregates [23]. It could also be due to recovery and regeneration of activity [25] or due to micro heterogeneity of covalently bound oligosaccharide residues at molecular levels [25] or still follow series-type inactivation kinetics [24]. In contrast, Morales- Blancas [25], observed monophasic inactivation kinetics for LOX from green asparagus at 70°C.
Figure 1: Semi log plots showing thermal inactivation of the three quality related enzymes at different temperatures. (1A) Peroxidase (POD), (1B) Polyphenol oxidase (PPO), and (1C) Lipoxygenase, (LOX). The enzymes in assay buffer were incubated at various temperatures. At regular intervals, aliquots were removed to measure the residual activity expressed relative to that of an unheated control.
POD appears to be comparatively, the most heat stable of the three quality-related enzymes studied. At 70°C, for a period of 80 min of incubation about 34% of the total enzyme activity was still active while in LOX and PPO only 10.71% and 11% residual activity was observed respectively for the same period (Figures 1a-c,). Soysal and Soylemez [28] reported that 50% of the total POD activity in carrots was heat resistant. In this report, at 30°C, only about 15% of the total POD activity was inactivated after 40min of incubation. For LOX, only 14.29% of enzyme activity was inactivated after 80 min of incubation at 30°C while studies on PPO showed that 17% of the total enzyme activity was inactivated at 30°C after 80min of incubation. At 40°C, 30.15% of total LOX activity was inactivated while 31% of PPO activity was inactivated at the same temperature and period of incubation. The result also shows that LOX is the most heat labile of the three, though it is possible that some of the lower estimates result from a failure to note nonlinearity in the Arrhenius plot along with the inclusion of data from a wide range of temperatures [1].
For the three enzymes studied, the semi-log plots of the residual enzyme activity versus heating time was observed to be non-linear at all the temperatures studied, suggesting that inactivation occurred by a simple first order model that assumes two isoforms of different thermal stabilities (Figures 2a-c).
The rate constants k1 and k2 for the first and second phase respectively, calculated from the slopes of plots of log% residual activity against time, increased logarithmically with temperature. The activation energy for inactivation, Ea, were obtained from the slopes of log k1 plotted against the inverse of absolute temperature (Figure 2), using the Arrhenius equation k=Ae-Ea/RT. For the first phase of the inactivation, larger values of Ea (2510 KJ/mol) were obtained for POD when compared to that of 2160 KJ/mol, obtained for PPO. Surprisingly, the Ea values obtained for lipoxygenase (2750 KJ/mol) was more than that for both peroxidase and polyphenol oxidase (Tables 1-3).
| k1 | k2 |
|---|---|
| Temp (oC) t1/2 (min) Ea (kJ/mol) ΔG (kJ/mol) ΔH (KJ/mol) ΔS (KJ/mol) D | t1/2(min) Ea(kJ/mol) ΔG(kJ/mol) ΔH(KJ/mol) ΔS(KJ/mol) D |
| 50 3.79 2510 45.82 -175.422 -221.24 51.2 | 4.14 2200 -296.98 -485.42 -188.44 72.0 |
| 60 3.40 2510 -37.29 -258.562 -221.27 34.4 | 3.89 2200 -380.09 -568.56 -188.47 56.2 |
| 70 2.86 2510 -120.37 -341.702 -221.30 20.2 | 3.18 2200 -463.2 -651.70 -188.50 27.8 |
| 80 2.05 2510 -203.51 -424.842 -221.33 8.9 | 2.67 2200 -546.31 -734.84 -188.53 16.6 |
| z-value = 14.11 | z-value = 19.46 |
Table 1: Half-life and activation parameters for the first and second phase (k1 & k2) respectively in the thermal inactivation of peroxidase.
| k1 | k2 |
|---|---|
| Temp (oC) t1/2 (min) Ea (kJ/mol) ΔG (kJ/mol) ΔH (KJ/mol) ΔS (KJ/mol) D | t1/2(min) Ea(kJ/mol) ΔG(kJ/mol) ΔH(KJ/mol) ΔS (KJ/mol) D |
| 50 3.43 2160 -304.12 -525.42 -221.30 35.4 | 3.94 1270 -1230.61 -1415.42 -184.81 59.1 |
| 60 2.62 2160 -387.23 -608.56 -221.33 15.9 | 3.59 1270 -1313.72 -1498.56 -184.84 41.9 |
| 70 2.28 2160 -470.34 -691.70 -221.36 11.2 | 3.51 1270 -1396.83 -1581.70 -184.87 38.4 |
| 80 1.90 2160 -552.95 -774.84 -221.39 7.7 | 3.01 1270 -1479.94 -1664.84 -184.90 23.3 |
| z-value = 8.78 | z-value = 11.09 |
Table 2: Half-life and activation parameters for the first and second phase (k1 & k2) respectively in the thermal inactivation of polyphenol oxidase.
| k1 | k2 |
|---|---|
| Temp(oC) t1/2(min) Ea(kJ/mol) ΔG(kJ/mol) ΔH(KJ/mol) ΔS(KJ/mol) D | t1/2(min) Ea(kJ/mol) ΔG(kJ/mol) ΔH(KJ/mol) ΔS(KJ/mol) D |
| 50 4.05 2750 279.01 64.58 -214.43 27.8 | 4.05 2160 -339.48 -525.42 -185.94 65.8 |
| 60 2.49 2750 195.77 -18.56 -214.33 14.0 | 3.07 2160 -422.59 -608.56 -185.97 24.8 |
| 70 1.67 2750 112.65 -101.70 -214.35 6.1 | 2.93 2160 -505.7 -691.70 -186.00 21.5 |
| 80 1.34 2750 29.54 -184.84 -214.38 4.4 | 2.44 2160 -588.82 -774.84 -186.02 13.2 |
| z-value = 7.81 | z-value = 16.11 |
Experimental data collected at 50oC. The values are given as SD from triplicates. Ea is the activation energy of inactivation process. It is obtained by plotting logk1, the first order inactivation constant, against reciprocal of temperature as per the Arrhenius equation k1 is obtained from the slopes of plots of log% residual activity against time in hours. The enzymes in assay buffer were incubated at various temperatures. At regular intervals, aliquots were removed to measure the residual activity expressed relative to that of the unheated control.
Table 3: Half-life and activation parameters for the first and second phase (k1 & k2) respectively in the thermal inactivation of lipoxygenase.
A larger value of Ea indicates that more energy is required to inactivate the enzyme. The free energy, (ΔG), enthalpy, (ΔH), and entropy (ΔS) of the activation at each temperature were calculated using equations (2), (3) and (4) respectively. The values obtained for the first and second phases are summarized in Tables 1-3. POD showed greater changes in free energy, enthalpy and entropy of inactivation when compared with PPO, but these two are much lower than that of lipoxyganase. In the case of the first phase ΔGvalue of POD were higher than PPO by 349.94 to 349.44 KJ mol-1. The enthalpy values did not vary significantly and the entropy values raised by 0.06 to 0.18 KJ mol-1 k-1. A similar trend in ΔG, ΔH, and ΔS, values are observed for the second phase. Nadege, et al. [5], observed activation energy of 67.67kJ mol-1 for PPO from edible yam (Dioscorea cayenesis- rotundata cv longbo). The differences observed might be as a result of the differences in the composition of the solution surrounding the enzyme during the heat treatment which could affect the kinetics of inactivation. The PPO here was heated in crude juice (pH 7.0), whereas the PPO by Nadege et al. [5], was at pH 6.6. The high Ea necessarily means that the rate of this process is strongly temperature dependent, and thus that at lower temperature, this rate becomes insignificant. At this lower temperature, then, the observed rate reflects some other process with much lower activation energy, such as the dissociation of heme or the loss of some other functional group. Both protein denaturation and loss of heme have been shown to be mechanism by which enzymes are inactivated.
The important observation from these thermoinactivation experiments (Tables 1-3), is that at higher temperatures, response to temperature would appear to follow a biphasic denaturation pattern suggesting that inactivation occurs by more than one mechanism each with its own temperature dependence.
The authors thank Mrs. Francisca O EZE of Dean’s Office Faculty of Biological Sciences, University of Nigeria for her encouragement in the research activities and for typing the manuscript.