Enzyme Engineering
Open Access
ISSN: 2329-6674
ISSN: 2329-6674
Editorial - (2012) Volume 1, Issue 2
Keywords: Tyrosinase, Molecular modeling, Copper (II) complexes, Enzyme action
Understanding the mechanisms of enzyme action is crucial in medical research to give appropriate therapies and help to identify individuals at greatest risk of drug interactions and adverse events [1]. Computational molecular modeling has been employed as a powerful tool to predict the events involved in molecular interactions such as molecular recognition, complexations and enzyme catalysis. Recently, Kamal and his colleagues at King Abdulaziz University started computational study of acetylcholinesterase interaction with two anticancer drugs, cyclophosphamide and methotrexate [2-4], while now collaborating with Ghalem and his team at University of Tlemcen on interaction between complexes of copper (II) and melanin pigment [5-9]
Following 8 previously reported complexes of copper have been selected for the current study:
1. 2 - { b i s [ ( 3 , 5 - d imé t h y l - 1H- p y r a z o l - 1 - y l ) ami n o } ethanoldichlorure de cuivre (II) monohydraté: [Cu(bp1mae) Cl]2,2[Cl.H2O] [10-12].
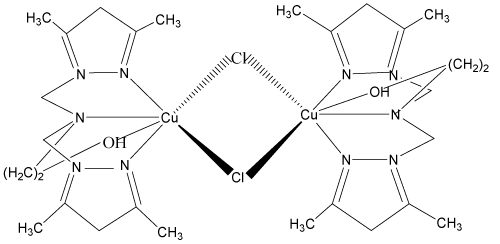
2. 2-{bis[(3,5-diméthyl-1H-pyrazol-1-yl)méthyl]amino} propanoldichlorure de cuivre (II) monohydraté: [Cu(bp1mapr) Cl]2, 2[Cl.H2O] [10-12].
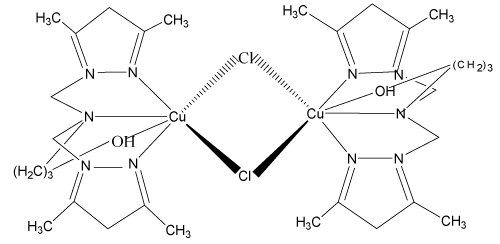
3. 2-{bis[(3,5-diméthyl-1H-pyrazol-1-yl)méthyl]amino} butanoldichlorure de cuivre (II) monohydraté: [Cu(bp1mab) Cl]2, 2[Cl.H2O] [10-12].
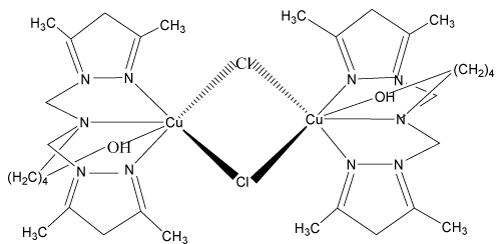
4. 2-{bis[(3,5-diméthyl-1H-pyrazol-1-yl)méthyl]amino} pentanoldichlorurede cuivre (II) monohydraté: [Cu(bp1map) Cl]2, 2[Cl.H2O] [10-12].
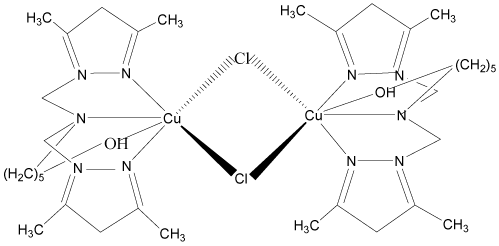
5. [N,N-bis(3,5-diméthyl-1H-pyrazol-1-ylméthyl-KN2)-2-hydroxyéthyl-amine-KN] nitratocuivre(II)nitrate: [Cu(bp1mae)L0(NO3)](NO3) [13-15].
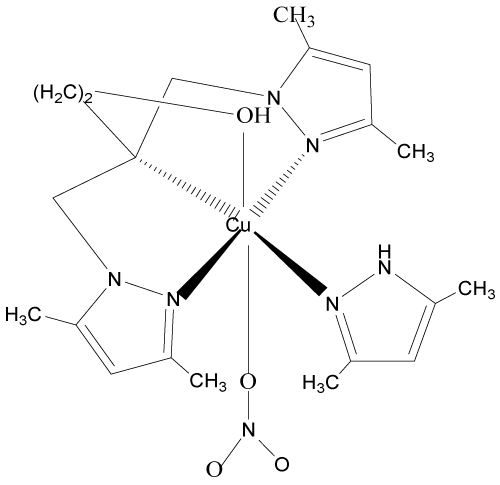
6. [N,N-bis(3,5-diméthyl-1H-pyrazol-1-ylméthyl-KN2)-2-hydroxypropyl-amine-KN] nitratocuivre(II)nitrate: [Cu(bp1mapr)L0(NO3)](NO3) [13-15].
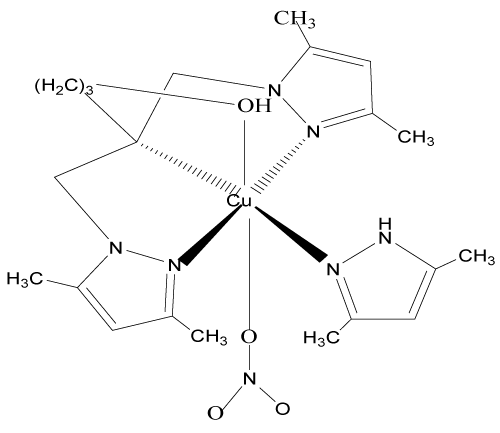
7. [N,N-bis(3,5-diméthyl-1H-pyrazol-1-ylméthyl-KN2)-2-hydroxybutyl-amine-KN] nitratocuivre(II)nitrate: [Cu(bp1mab)L0(NO3)](NO3) [13,14,15].
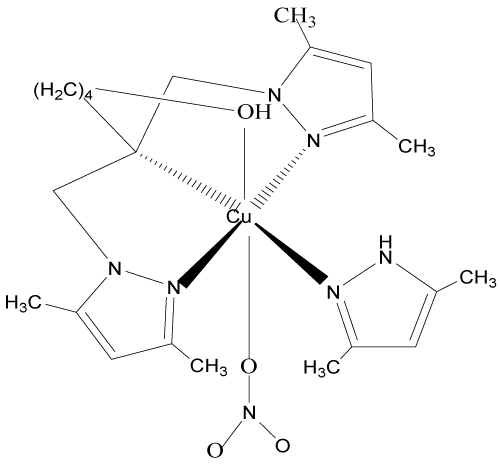
8. [N,N-bis(3,5-diméthyl-1H-pyrazol-1-ylméthyl-KN2)-2-hydroxypentyl-amine-KN] nitratocuivre(II)nitrate: [Cu(bp1map)L0(NO3)](NO3) [13,14,15].
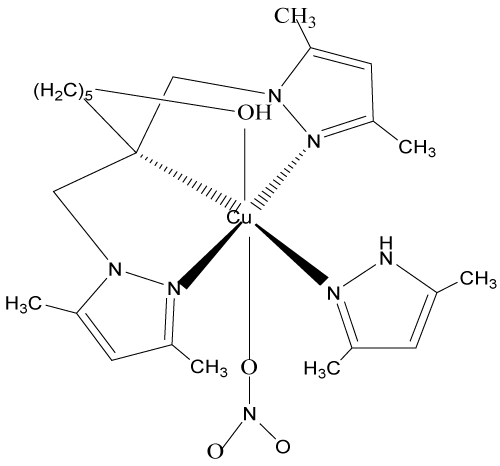
The theoretical binding energies of the above complexes were calculated using EMO (Energy of molecule) program; Version 2010 [16,17] based on the principle of force field MM2. In this relaxation method of molecular mechanical calculations, EMO uses a truncated parabolic potential, equal to -16.7 KJ per bond at the optimal distance of 1.85Aº, and to zero at 1.25 or 2.45 Aº. The program protocol includes an entry of the molecule in which atoms are codified according to its hybridization, geometric handling of the submitted molecule and followed by energy minimization by molecular mechanics. The most stable conformation is obtained from various geometries, after optimization. In order to avoid the local minima corresponding to unstable conformers, we carried out it with the option ‘‘SCAN’’ which makes it possible to sweep the surface of potential energy (PES). This enabled us to eliminate the geometries having little chance to generate the most stable conformation.
Gaussian09 program [18], also used to calculate theoretical binding energies of copper complexes containing pyrazole II and to explain the influence of the length of the side chain on their stability. The data generated from EMO and Gaussian09 program has been analyzed and compared with the experimental data obtained from Mohamed Laboratory.
Influence of the lateral chain on the stability of the complexes
Chloride complexes: These complexes activate the oxidation reaction of catechol to o-quinone with different re-activities as shown by the results reported in the following table 1.
| Complexes | Carbon number(n) | Catalyticactivity(υ) (μ mol / mg / min) |
|---|---|---|
| [Cu(bp1mae)Cl]2,2[Cl.H2O] | 2 | 0.16 |
| [Cu(bp1mab)Cl]2,2[Cl.H2O] | 4 | 1.77 |
| [Cu(bp1map)Cl]2,2[Cl.H2O] | 5 | 2.67 |
Table 1: Kinetic data of Chloride complexes [19].
Experimental results: Rate varying from a high 2.67 μmole substrate per mg catalyst per min for n=5 complex, the catalytic activities depend strongly on the length of the lateral chains [19].
Theoretical results: In the case of anion Cl ¯ for which the complex ligands having a rather long lateral chain (4 or 5 carbon) have the lowest energy but for the chains of n=2 or 3 in this case the Cl ¯ forms the strong bonds with the cation and the oxygen of the side chain and forms a cycle of 5 or 6-chelating atoms stable [20], and find difficulties in the coordination of the substrate which explains the low catalytic activity of this type of complex. With a longer chain of 4 or 5 carbons, the cycle of chelation of 7 or 8 atoms is much less stable [20] allow the substrate to coordinate to the metal without difficulty which leads to a significant catalytic activity (Table 2).
| Complexes | Carbon number (n) | Energy (kJ / mol) EMO | Energy (KJ/mol) Gaussian |
|---|---|---|---|
| [Cu(bp1mae)Cl]2,2[Cl.H2O] | 2 | 1739.83 | 1501.24 |
| [Cu(bp1mapr)Cl]2,2[Cl.H2O] | 3 | 2020.97 | 1955.47 |
| [Cu(bp1mab)Cl]2,2[Cl.H2O] | 4 | 2304.38 | 2090.57 |
| [Cu(bp1map)Cl]2,2[Cl.H2O] | 5 | 2709.66 | 2486.74 |
Table 2: Energies (kJ/mol) obtained using the program Gaussian09 and EMO.
Nitrate complex
Experimental results: Rate varying from a high concentration of 0.08 μmol substrate per mg catalyst per min for the n=4 complex the catalytic activities depend strongly on both the length of the lateral chains [19] (Table 3).
| Complexes | Carbon number (n) | Catalytic activity (υ) (μ mol/mg/min) |
|---|---|---|
| [Cu(bp1mae)L0(NO3)](NO3) | 2 | 0.01 |
| [Cu(bp1mab)L0(NO3)](NO3) | 4 | 0.08 |
| [Cu(bp1map)L0(NO3)](NO3) | 5 | 0.03 |
Table 3: Kinetic data of Nitrate complex [19].
Theoretical results: In light of the results for both types of complexes it was noticed that the steric energy, the energy of van der walls, the torsional energy, the strain energy and the energy of elongation increases with the number carbon of the side chain. This can be explained by the steric hindrance of this latter. Higher the carbon numbers, the more the congestion is important. According to the literature, a series of chelation of four or five-member cycle is more stable than six-member chelating itself and more stable than a seven-member chelate cycle [20]. The complex which acts as a good catalyst is the less stable and dissociate easily (Table 4).
| Complexes | Carbon number (n) | Energy (KJ/mol) EMO | Energy (KJ/mol) Gaussian09 |
|---|---|---|---|
| [Cu(bp1mae)Cl]2,2[Cl.H2O] | 2 | 566,84 | 471,05 |
| [Cu(bp1mapr)Cl]2,2[Cl.H2O] | 3 | 606,25 | 507,28 |
| [Cu(bp1mab)Cl]2,2[Cl.H2O] | 4 | 676,42 | 631,75 |
| [Cu(bp1map)Cl]2,2[Cl.H2O] | 5 | 795,72 | 691,24 |
Table 4: Energies (kJ/mol) obtained using the program EMO and Gaussian 09.
Experimental results (Table 5)
| Carbon number (n) | Catalytic activity (υ) of Chlorure complexe (μ mol / mg / min) | Catalytic activity (υ) of Nitrate complexe (μ mol / mg / min) |
|---|---|---|
| 2 | 0.16 | 0.01 |
| 4 | 0.77 | 0.08 |
| 5 | 2.67 | 0.03 |
Table 5: Kinetic data Comparison between the complexes of Chloride and Nitrate complexes [19].
Theoretical results (Table 6)
| Carbon number (n) | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| Energy (KJ/mol) of Chlorure complexe | 1501,24 | 1955,47 | 2090,57 | 2486,74 |
| Energy (KJ/mol) of Nitrate complexe | 471,05 | 507,28 | 631,75 | 691,24 |
Table 6: Energies (kJ/mol) obtained using the program Gaussian 09.
Based on the results presented in the current study in the tables and figures, we observed that the nature of the anion plays a very important role. By comparing the results, we found that our two kinds of complexes have similar results as obtained by Boyd et al. [21]. We note that chloride complexes are much less stable than the nitrate complex, so the chloride complexes react better as catalysts, which explains the catalytic activity of these complexes. This factor may contribute in the explanation of the dependence of the catalytic activity of the oxidation of catechol to quinone of the anion nature (Figure 1).
Based on steric energies of different complexes studied, the current study demonstrated the difference in catalytic activities of 8 different complexes, which depends on the size of the side chain carrying the hydroxyl group. The complexes of ligands having a side chain rather long (4 or 5 carbons) provide a catalytic activity greater than the complex ligands of having only two carbons on the side chain. Since the ions NO3- and Cl- form bonds with strong enough with the cation and oxygen of the side chain and forms a ring having 5 atoms chelating stable will allow the substrate to coordinate to the metal without difficulty, which leads to important catalytic activity. These results shows the difference in stability of each copper ion-ligand complex as present in the enzyme tyrosinase and which might influence enzyme activity and synthesis of melanin and other pigments. Even though this work has some limitations as not been assisted with potentiometric analysis, this interesting results could lead to new developments in synthetic biochemistry such as generating more stable conformers.