Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2024)Volume 9, Issue 5
Object: To study how Gut Microbiota (GM) in Idiopathic Central Precocious Puberty (ICPP) children affects Hyperandrogenemia (HA) and Insulin Resistance (IR).
Methods: In this study, we have recruited 27 ICPP (ICPP group) and 23 healthy children (healthy group) and collected the blood and fecal samples from the participants. Blood samples were tested for hormones, including the follicle-stimulating hormone, luteinizing hormone, estradiol, prolactin and testosterone. Deoxyribonucleic Acid (DNA) was extracted from fecal samples and amplified and sequenced with 16S Ribosomal DNA (16S rDNA) Variable (V3-V4) region. Finally, we annotated the sequencing results, counted the differences in hormone indicators and GM composition between the two groups and analysed the correlation with clinical indicators.
Results: 1. Compared with the healthy group, hormone levels such as Androstenedione (A2), insulin, insulin resistance index and Insulin-like Growth Factor 1 (IGF-1) in the ICPP group were significantly higher (p<0.05); 2. At the phylum level, the ICPP group showed significantly enriched Proteobacteria than the healthy group (4.85% vs. 2.92%); 3. At the genus level, the abundances of Roseburia and Prevotella were significantly higher in the ICPP group than those in the healthy group (7.55% vs. 2.01%, 3.95% vs. 0.19%), but Bacteroides were obviously decreased in the ICPP group (29.96% vs. 44.91%).
Conclusion: Changes in GM promote HA and IR, the latter may be an important pathogenesis in ICPP children.
Idiopathic central precocious puberty; Gut microbiota; Hyperandrogenemia; Insulin resistance
Precocious Puberty (PP) refers to girls before the age of 8 and boys before the age of 9 with the second sexual sign. Central Precocious Puberty (CPP) is defined as the onset of the Hypothalamus-Pituitary-Gonadal Axis (HPGA) function and Idiopathic Central Precocious Puberty (ICPP) is the most common. At present, the pathogenesis of ICPP is unknown. The main drug for the treatment of CPP is acetate gonadotropin-releasing hormone analogue. It must be intramuscularly injected every 4 weeks. The treatment cost is expensive and the treatment time is long. It is necessary to find another treatment as an alternative or auxiliary treatment. It is well known that CPP is associated with genes that affect the development of HPGA. Genes play an important role in the development and regulation of HPGA, the migration and secretion of embryonic Gonadotropin-Releasing Hormone (GnRH) neurons and the regulation and role of hypothalamic GnRH. In CPP patients, stimulated by GnRH secretion, Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH) secretion pulse and peak also increased, especially LH, LH/FSH ratio increased, high LH directly acted on thecal cells, increased the activity of intracellular straight-chain lyase, stimulated ovarian follicle cells to synthesize androgen early and hyper function, resulting in ovarian derived androgen and LH also induced ovarian synthesis Insulin Like Growth Factor-1 (IGF-1) receptor which can increase its binding amount, induce the proliferation of thecal cells and promote the synthesis and secretion of ovarian androgen [1]. CPP is the result of premature activation of the HPGA which affects pubertal gonadotropin levels and progressive characteristics associated with linear growth acceleration and late bone age.
In recent years, the morbidity of CPP has increased significantly. The morbidity of CPP is 5.66/100 million worldwide per year and most of which are ICPP [2]. Studies have found that PP children have dietary structure changes [3-5]. Interesting, diet is closely related to GM and GM also plays a crucial role in food digestion, nutrition absorption, nutrition metabolism, immune function activation, intestinal barrier and body behaviour regulation, which is recognized as an important "microbial organ" of the human body [6]. Studies have also found that PP children have HA and IR [7-12]. Is there any correlation between changes in GM of ICPP children and insulin resistance and hyperandrogenism? In this study, we recruited 27 ICPP children and 23 healthy age-matched individuals, investigate their diet, detected their GM compositions and analysed the correlations between the GM compositions, GM functions and the clinical indicators such as endocrine hormones, explore whether changes in GM can lead to HA and IR.
Ethics statement
The study was approved by the Longgang District Maternity and Child Healthcare Hospital Ethics under Committee number: (2019) LGFYYXLL-024. All procedures were performed following relevant guidelines and regulations stipulated by the Ethics Committee. Before the study, all the parents of the children filled in the written informed consent form and the parents agreed their children participate in the study.
Research object
From July 1 to December 31 in 2019, 27 children with ICPP diagnosed in the child health care department of Longgang District Maternity and Child Healthcare Hospital were selected as the disease group, referred to as ICPP group; another 23 healthy children of the same age who underwent physical examination in the child health care department were recruited as the healthy group. The children in both groups were girls, aged between 6 and 10 years old. The ICPP diagnosis and inclusion criteria as following (12); all patients had a secondary sexual sign before 8 years old, or menarche before 10 years old, ovarian volume>1 ml, multiple follicles with diameter>4 mm, GnRH stimulation test LH>5 IU/L, LH/FSH>0.6 (chemiluminescence method), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) excluded central tumor and injury, as well as other organic diseases. All the children did not use antibiotics and had no gastrointestinal symptoms such as diarrhoea two weeks before fecal collection.
Clinical observation index
The clinical data of all enrolled children were investigated by a specially assigned person. The height and weight were measured and the Body Mass Index (BMI) was calculated:
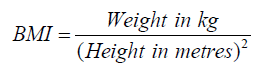
Blood samples were collected and sent to Guangzhou Jinyu Inspection Center, testing of FSH, LH, fasting insulin, fasting blood glucose and Androstenedione (A2) by chemiluminescence method; IGF-1 was detected by tandem mass spectrometry and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index was calculated.
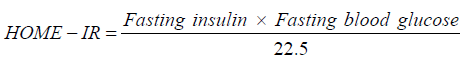
Fecal sample collection and DNA sequencing
Fecal samples from the ICPP and the healthy children group were collected at the same period of date. About 5 g fecal were sampled from the middle part of feces. The fecal samples were frozen immediately after collection, stored in -80°C and transported to Beijing novogene Technology Co., Ltd. for 16S rDNA sequencing. All the bacterial DNA was extracted from fecal samples using the PowerSoil® DNA Isolation Kit. The V3-V4 region of 16S rRNA gene was amplified by Polymerase Chain Reaction (PCR) and the amplified sequences were sequenced using Illumina Miseq.
Data analysis
The 16S rDNA sequencing data were filtered by self-designed bioinformatics tools and Flash software (v1.2.11, http://ccb.jhu.edu/software/FLASH/index.shtml) was used to connect the paired-end reads. Then the connected tags were clustered into Operational Taxonomic Units (OTUs) by usearch. The OTUs were annotated with the Greengene database (V201305) and their relative abundances were calculated for all samples. The differentially enriched bacteria between the ICPP and healthy groups were analyzed at the phylum, class, order, family and genus levels.
Statistical methods
The ADE4 software package of R (v3.3.3) was used to analyze the compositions and relative abundances of genus in all samples. Principal Components Analysis (PCA) was carried out based on the profiling results and the overall microbiota distribution of the ICPP and healthy groups was exhibited. Statistical Package for the Social Sciences (SPSS 23.0) was used for statistical analysis. Age, weight, height and endocrine hormone values of the two groups were compared by two independent sample t-test and the chi-square test was used for sweet food and high fat in the diet survey. p<0.05 was considered to be statistically significant.
Clinical indicators
Under the same averaged age, the weight, height, BMI, number of sweet eaters and number of high-fat eaters in the ICPP group were significantly higher than those in the healthy group (p<0.05) (Table 1). The levels of serum FSH, LH, estradiol, serum insulin, IGF-1, A2, fasting blood glucose and HOMA-IR were significantly higher in the ICPP group than those in the healthy children (p<0.05) (Table 1).
| Characteristics | ICPP (N=27) | Healthy(N=23) | p/χ²-value |
|---|---|---|---|
| Age (mean ± SD, year) | 7.72 ± 0.45 | 7.4 ± 0.77 | 0.204 |
| Height (mean ± SD, cm) | 138.89 ± 6.36 | 129.87 ± 6.39 | 0 |
| Weight (mean ± SD, kg) | 34.92 ± 6.92 | 26.31 ± 5.29 | 0 |
| BMI (mean ± SD) | 18.05 ± 2.97 | 15.64 ± 3.10 | 0.008 |
| Number of sweet eaters (n) | 19/27 | 8/23 | 0.012 |
| Number of high-fat eaters (n) | 17/27 | 8/23 | 0.047 |
| FSH (mean ± SD, mIU/ml) | 4.02 ± 1.75 | 2.09 ± 1.02 | 0 |
| LH (mean ± SD, mIU/m) | 1.39 ± 1.17 | 0.17 ± 0.21 | 0 |
| E2 (mean ± SD, pmol/L) | 27.44 ± 18.05 | 2.61 ± 8.80 | 0.001 |
| Insulin (mean ± SD,μU/mL) | 10.07 ± 5.52 | 5.114 ± 3.78 | 0 |
| A2 (mean ± SD,μg/L) | 2.06 ± 0.37 | 0.73 ± 0.42 | 0 |
| IGF-1 (mean ± SD, ng/mL) | 308.19 ± 54.42 | 205.66 ± 49.00 | 0 |
| BS (mean ± SD, mmol/L) | 4.85 ± 0.79 | 4.35 ± 0.64 | 0.018 |
| HOMA-IR (mean ± SD) | 2.23 ± 1.38 | 1.05 ± 0.89 | 0.01 |
Note: SD: Standard deviation; BMI: Body mass index; FSH: Follicle stimulating hormone; LH: Luteinizing hormone; E2: Estradiol; A2: Androstenedione; IGF-1: Insulin-like growth factor 1; BS: Blood sugar; HOMA-IR: Homeostatic Model assessment for insulin resistance index.
Table 1: The clinical information of the Idiopathic Central Precocious Puberty (ICPP) and healthy girls.
The difference of GM in ICPP girls
Analysis of dominant bacteria and their difference at the phylum level: We analysed the GM compositions in 50 samples from the ICPP and healthy groups (Table 2). At the phylum level, Firmicutes accounted for 52.75%, Bacteroides 38.54%, Proteobacteria 4.85%, Actinobacteria 2.88%, Fusobacteria 0.18% in the ICPP group. Meanwhile, Firmicutes accounted for 45.81%, Bacteroides 46.95%, Proteobacteria 2.92%, Actinobacteria 2.59%, Fusobacteria 1.65% in the healthy group. There were statistically significant differences in Proteobacteria and Fusobacteria between the two groups (p<0.05).
| The top 5 phyla | ICPP (N = 27) | Healthy (N = 23) | P-value | FDR-value |
|---|---|---|---|---|
| Firmicutes | 52.75 ± 11.61 | 45.81 ± 18.27 | 0.1348 | 0.2023 |
| Bacteroidetes | 38.54 ± 12.92 | 46.95 ± 19.22 | 0.1024 | 0.1843 |
| Proteobacteria | 4.85 ± 2.58 | 2.92 ± 1.86 | 0.0031 | 0.0094 |
| Actinobacteria | 2.88 ± 1.78 | 2.59 ± 3.03 | 0.0731 | 0.1646 |
| Fusobacteria | 0.18 ± 0.33 | 1.65 ± 4.77 | 0.3003 | 0.3378 |
Table 2: The top 5 dominant bacteria at phylum level in Idiopathic Central Precocious Puberty (ICPP) and healthy groups (difference screening conditions; average relative abundance>0.1%, p<0.05, False Discovery Rate (FDR)<0.05).
Detection of the dominant genera and the differentially enriched genera: At the genus level, we selected the top 18 dominant genera from each group and counted their proportions respectively (Table 3). In the ICPP group, Bacteroides accounted for 29.96%, Roseburia accounted for 7.55%, Prevotella accounted for 3.95%. Whilst, in the healthy group, Bacteroides accounted for 44.91%, Megamonas accounted for 5.25% and Roseburia accounted for 2.01%. We listed the top 18 abundant genera, the dominant genera with significant differences between the two groups included Bacteroides, Roseburia and Prevotella. In addition, the abundance of conditional pathogens such as Alipipes, Klebsiella and Sutterella in ICPP children significantly higher than the healthy group (Table 3).
| The top 18 dominant bacteria | ICPP (N=27) | Healthy (N=25) | p-value | FDR-value |
|---|---|---|---|---|
| Bacteroides (mean ± SD, %) | 29.96 ± 10.77 | 44.91 ± 19.80 | 0.0016 | 0.0033 |
| Roseburia (mean ± SD, %) | 7.55 ± 3.15 | 2.01 ± 2.93 | 0 | 0 |
| Prevotella (mean ± SD, %) | 3.95 ± 4.81 | 0.19 ± 0.87 | 0 | 0 |
| Lachnospiracea incertae (mean ± SD, %) | 3.36 ± 1.63 | 1.53 ± 2.07 | 0.0003 | 0.0009 |
| Ruminococcus (mean ± SD, %) | 2.50 ± 1.00 | 0.73 ± 1.27 | 0 | 0.0001 |
| Parabacteroides (mean ± SD, %) | 2.39 ± 1.14 | 1.42 ± 1.79 | 0.0004 | 0.0012 |
| Alistipes (mean ± SD, %) | 1.73 ± 0.52 | 0.28 ± 0.48 | 0 | 0 |
| Ruminococcus2 (mean ± SD, %) | 1.20 ± 0.44 | 0.80 ± 0.82 | 0.005 | 0.0089 |
| Fusicatenibacter(mean ± SD, %) | 1.16 ± 0.55 | 1.13 ± 2.46 | 0.0013 | 0.0031 |
| Clostridium sensu stricto (mean ± SD, %) | 0.98 ± 1.26 | 0.53 ± 1.93 | 0 | 0.0001 |
| Clostridium XVIII(mean ± SD, %) | 0.85 ± 1.32 | 0.23 ± 0.48 | 0 | 0 |
| Megamonas(mean ± SD, %) | 0.79 ± 0.56 | 0.52 ± 1.28 | 0.0037 | 0.0067 |
| Parasutterella(mean ± SD, %) | 0.77 ± 0.56 | 0.71 ± 1.27 | 0.0132 | 0.0201 |
| Gemmiger(mean ± SD, %) | 0.74 ± 0.60 | 0.76 ± 1.83 | 0.0003 | 0.001 |
| Klebsiella(mean ± SD, %) | 0.72 ± 0.85 | 0.10 ± 0.26 | 0.0008 | 0.002 |
| Sutterella(mean ± SD, %) | 0.22 ± 0.20 | 0.53 ± 1.24 | 0.002 | 0.004 |
| Megasphaera(mean ± SD, %) | 0.30 ± 0.33 | 0.52 ± 1.28 | 0.0019 | 0.0038 |
| Clostridium IV(mean ± SD, %) | 0.38 ± 0.19 | 0.42 ± 0.96 | 0.0003 | 0.0009 |
Note: SD: Standard deviation
Table 3: The top 18 dominant bacteria at genus level in Idiopathic Central Precocious Puberty (ICPP) and healthy groups (difference screening conditions: average relative abundance>0.1%, p<0.05, False Discovery Rate (FDR)<0.05).
PCA analysis on the GM at the genus level
The GM similarities between the samples from the two groups were analysed by PCA. According to the distributions of samples, children from the ICPP group were clustered together and clear boundaries existed between the two groups (Figure 1).
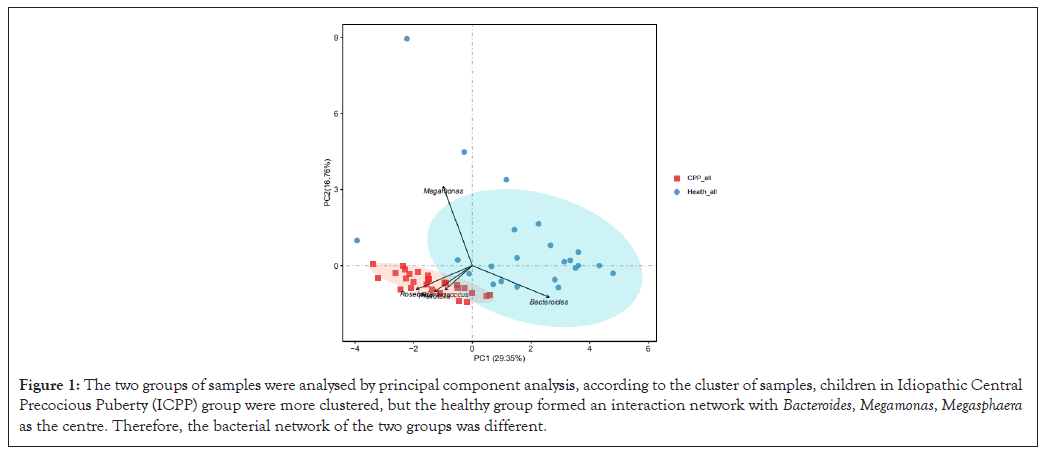
Figure 1: The two groups of samples were analysed by principal component analysis, according to the cluster of samples, children in Idiopathic Central Precocious Puberty (ICPP) group were more clustered, but the healthy group formed an interaction network with Bacteroides, Megamonas, Megasphaera as the centre. Therefore, the bacterial network of the two groups was different.
Analysis of GM, signal transduction and nutrition metabolism
Due to the GM differences, the functional distributions of GM also different between the ICPP and healthy groups, including the fructose synthesis and metabolism, genetic information processing, nucleotide metabolism, cell process and signal transmission, energy metabolism and carbohydrate metabolism. Particularly, the GM in the ICPP group contained the enhanced carbohydrate metabolism (Figure 2).
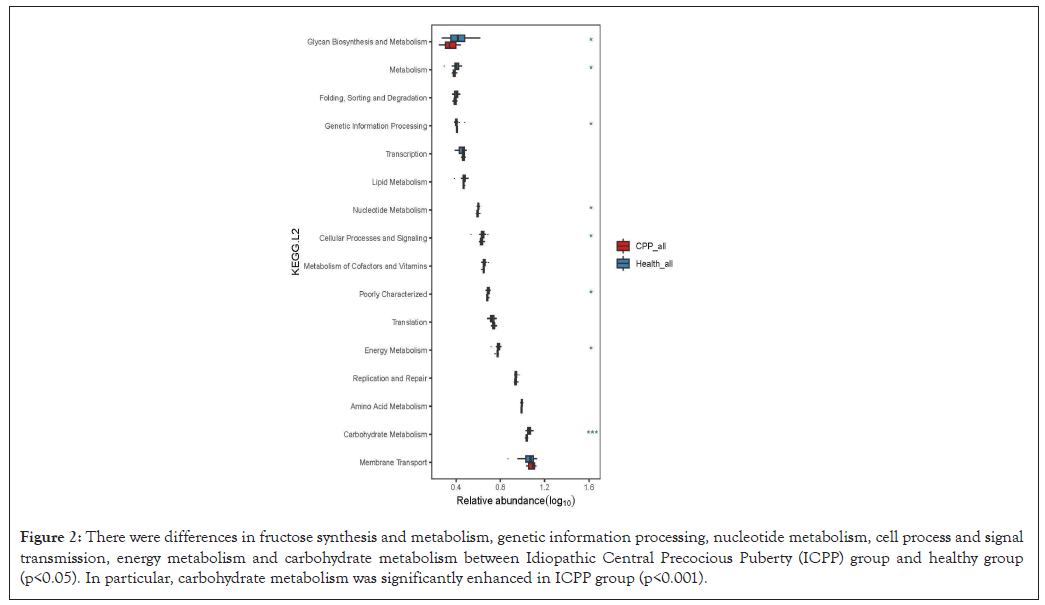
Figure 2: There were differences in fructose synthesis and metabolism, genetic information processing, nucleotide metabolism, cell process and signal transmission, energy metabolism and carbohydrate metabolism between Idiopathic Central Precocious Puberty (ICPP) group and healthy group (p<0.05). In particular, carbohydrate metabolism was significantly enhanced in ICPP group (p<0.001).
The associations between GM and hormones
Performing the correlation analysis between the GM and the clinical hormone levels in the ICPP children, we discovered that the bacteria with higher abundance (e.g. Roseburia, Prevotella, Clostridridium sensu stricto) were significantly associated with the levels of FSH, Estradiol (E2) and A2, while Sutterella was significantly associated with the above hormone levels in healthy group (Figure 3).
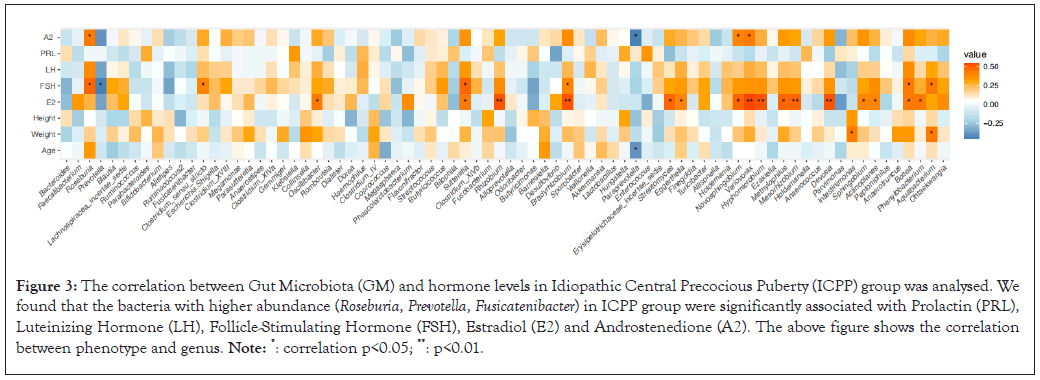
Figure 3: The correlation between Gut Microbiota (GM) and hormone levels in Idiopathic Central Precocious Puberty (ICPP) group was analysed. We found that the bacteria with higher abundance (Roseburia, Prevotella, Fusicatenibacter) in ICPP group were significantly associated with Prolactin (PRL), Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH), Estradiol (E2) and Androstenedione (A2). The above figure shows the correlation between phenotype and genus. Note: *: correlation p<0.05; **: p<0.01.
Wang et al., reported, high-glucose and high-fat conditions promote the secretion of GnRH and Estrogen Receptors (ER) and the expression of genes related to sexual precocity in GT1-7 cells [13]. Hernnandez et al., found that both high-sugar and high-fat diets can induce obesity and hyperleptinemia, but high-sugar can also affect insulin secretion, oxidative stress, etc [14]. The above research shows that a high-sugar and high-fat diet is closely related to PP, our study also found that children in the ICPP group preferred sweet food (high-sugar diet) and high-fat food. We know that high-sugar diet belonged to low molecular carbohydrate. Moreover, we found the abundance of bacteria such as Prevotella that carbohydrate metabolizing in GM was significantly increased and carbohydrate metabolism in GM was significantly enhanced with the ICPP group. combined with increased insulin levels and insulin resistance in our investigation, we speculate that high-sugar diet may be one of the high risk factors for ICPP children.
Analysis of GM composition and function in ICPP children
The results of the study suggest that GM of ICPP children has changed significantly. On the one hand, the abundance of butyric acid-producing bacteria such as Roseburia, Prevotella, Lachnospiracea incertae sedis, Ruminococcus are significantly increased and the production of Short-Chain Fatty Acids (SCFAs) such as butyrate is increased, SCFAs helps to maintain the homeostasis of the gut microenvironment and butyrate can directly stimulate the secretion of glucagon-like peptide-1 (GLP-1) by endocrine cells GPR43 or GPR41 in colon and ileum, can also promote the synthesis of neurotransmitters such as Nitric Oxide (NO) and insulin secretion [15-21]. On the other hand, the abundance of conditional pathogenic bacteria such as Parabacteroides, Alistipes, Klebsiella and Sutterella in ICPP children increases, can cause the reduction of the synthesis of tight junction protein (tight junction protein-1 and atresia protein), thus leading to the occurrence of "gut leakage" and intestinal inflammation [22-24]. In addition, Clostridium scindens can produce testosterone, Fusobacterium, was positively correlated with FSH [25,26]. We also found that the bacteria with higher abundance (e.g. Roseburia, Prevotella, Clostridridium sensu stricto) are related to the levels of FSH, E2 and A2. Therefore, we can speculate that changes in GM of ICPP children can affect the abnormal production of SCFAs, chronic inflammation in the intestine and even affect the secretion of sex hormones.
GM and HA
Studies have also found that PP children have hyperandrogenemia [7,8]. The negative feedback of hyperandrogenemia caused the decrease of estradiol and progesterone secretion and the increase of GnRH and LH levels [27]. androgen in women secreted by the ovarian tissue. The testosterone and A2 secreted by the ovary account for two-thirds of the circulating androgens [28]. The androgen synthesis in the ovary is regulated by LH. Total serum testosterone is the most common and widely used biochemical test index to evaluate androgen excess [29]. However, there are many defects in the detection method of testosterone in clinical practice. A2 expression is closely related to endocrine and metabolic disorders, so A2 is increasingly used to assess androgen levels [30,31]. In this project, A2 in the ICPP group was significantly higher than that in healthy group, which may be caused by the following factors. According to the investigation, the children in the ICPP group like to eat sweet food (carbohydrate intake is more) and the correlation analysis between GM and hormone level of children also indicates that the metabolism of carbohydrate in children in the ICPP group is significantly higher than that in the healthy group and high sugar diet can cause an immune response and oxidative stress to produce inflammatory mediators and stimulate ovaries to produce androgen [32]. Compared with healthy children, the bacterial abundance of butyric acid-producing bacteria in the ICPP group was significantly increased, butyric acid-induced insulin secretion through GLP-1, insulin enhanced GnRH gene transcription by enhancing mitogen-activated protein kinase pathway and increased GnRH secretion in the hypothalamus, resulting in high levels of androgen and LH secretion [27,33]. On the other hand, insulin can improve the utilization of IGF-1 by inhibiting the expression of IGFBP-1 in the ovary and liver. Insulin can also improve IGF-1 activity in the liver and ovary, reduce the level of Sex Hormone Binding Globulin (SHBG) in the liver and ovary and increase free androgen in vivo [27,34]. In the ICPP group, the abundance of butyric acid-producing bacteria such as Roseburia, Prevotella, Lachnospiracea incertae sedis and Ruminococcus increased, which may lead to the rich energy of intestinal mucosal cells, the secretion of Adrenocorticotropic Hormone (ACTH) and other hormones in the gut tract and also affect the HPGA to promote the synthesis of androgen [34]. The abundance of Clostridium sensu stricto and Clostridium XVIII in the ICPP group is increased, the latter can convert glucocorticoid into androgen, studies have confirmed that the bacterium can produce testosterone [25,35]. 60% of testosterone in women is converted from its direct precursor A2.
GM and IR
Studies have also found that PP children have insulin resistance [8-11]. In this project, insulin level, IGF-1 and IR in the ICPP group was significantly higher than that in healthy group, which may be caused by the following factors. The increase of androgen level can cause gut mucosal damage (337) and the abundance of Parabacteroides, Alistipes, Klebsiella and Sutterella increased can lead to gut inflammation [22-24,36]. Increased intestinal permeability causes "gut leakage", many inflammatory mediators produced by GM, such as Lipopolysaccharide (LPS), can activate the immune response and stimulate the production of various inflammatory factors by activating Toll-Like Receptors 4 (TLR4), through Nuclear Factor-κB (NF-κB) and other signalling pathways, it can promote the phosphorylation of Insulin Receptor Substrate-1 (IRS-1) in the insulin signalling pathway, resulting in the decrease of insulin sensitivity [37]. The abundance of Roseburia, Prevotella, Lachnospiracea incertae sedis, Ruminococcus increased can lead to increased production of SCFAs such as butyrate, the latter can stimulate the increase of GLP-1 content, promote insulin secretion [27,33]. Our previous study also found that ICPP children have increased NO synthesis in GM, which is positively correlated with FSH and insulin levels and NO can promote insulin resistance [38]. Prevotella abundance was increased in ICPP children in our study and Prevotella could increase circulating Branched-Chain Amino Acid (BCAA) level, the latter was positively correlated with free testosterone level and insulin resistance [39,40].
HA and IR cause neuroendocrine abnormalities of adrenal glands and ovaries
HA can reduce the sensitivity and expression level of Glucose Transporter 4 (Glu-4), inhibit the degradation of insulin by the liver and aggravate central obesity, it is an important potential mechanism of IR [41]. IR can enhance the regulation effect of upstream LH on the ovary and strengthen the function of the adrenal gland to synthesize androgen; it can also inhibit the synthesis of SHBG in the liver, reduce the binding of androgen and SHBG in serum and cause the increase of free testosterone level, cause HA [37]. HA and IR cause neuroendocrine abnormalities in the adrenal glands and ovaries through the following two ways. Lead to the imbalance of Hypothalamic-Pituitary-Adrenal (HPA) axis regulation, increase the secretion of GnRH, LH, FSH. Lead to the imbalance of Hypothalamic-Pituitary-Ovary (HPO) axis regulation, increase the secretion of Corticotropin Releasing Hormone (CRH) and ACTH [42]. The secretion of the above hormones can start HPGA in advance and promote the occurrence and development of ICPP.
Excessive intake of high-sugar and high-fat diets in ICPP children, especially high-sugar foods, may lead to changes in GM. Abnormal metabolites of GM induce HA and IR. The latter affect HPA and HPO, leading to abnormal secretion of GnRH, CRH, ACTH, etc. in the hypothalamus, promote the secretion of FSH, LH, insulin, A2 and other hormones, thereby starting HPGA in advance, which may be an important pathogenesis of ICPP. The sample size of this study is small and the faecal samples are only for 16S rRNA sequencing. The next step is to carry out the large sample and multi-centre research. It is better to take macro genre sequencing for faecal samples, combined with the analysis of GM metabolites and blood metabolites, which may be more helpful to clarify the pathogenesis of ICPP.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Han J, Huang C, Chen J, Chen S, Wu B, Meng D (2024). Study on Hyperandrogenemia and Insulin Resistance in Children with Idiopathic Central Precocious Puberty Based on Gut Microbiota. Clin Pediatr. 09:278.
Received: 02-Aug-2024, Manuscript No. CPOA-24-33325; Editor assigned: 05-Aug-2024, Pre QC No. CPOA-24-33325 (PQ); Reviewed: 19-Aug-2024, QC No. CPOA-24-33325; Revised: 26-Aug-2024, Manuscript No. CPOA-24-33325 (R); Published: 02-Sep-2024 , DOI: 10.35248/2572-0775.24.09.278
Copyright: © 2024 Han J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.