Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
+44 1300 500008
ISSN: 2157-7064
+44 1300 500008
Research Article - (2012) Volume 3, Issue 5
This research studied the remove of lead metal and natural organic matter (NOM) using nanofiltration membrane. The combined effects between NOM and lead ion can cause a loose cake formation at the membrane surface, thus lowering flux decline when compared with that of metal ion alone. The results were possibly caused by increased accumulation of fouled materials at the membrane surface, thus affecting flux, causing complete pore fouling.
Keywords: Removal; Lead; Natural organic matter; Nanofiltration; Fouling
Jv: volumetric flux (L.m-2 h-1); Qp: Permeate flux (m3/h); LP: hydraulic permeability of the membrane (m.h-1KPa-1); ΔP: Transmembrane hydraulic pressure (KPa); ΔΠ: Transmembrane osmotic pressure (KPa); Am: Membrane area (m2); Cp: Permeate concentrate salt (mg/L); Cf: feed concentrate salt (mg/L); %R: % Rejection; Pf: Feed pressure (KPa); Pp: Permeate pressure (KPa); Pc: Concentrate pressure (KPa); Greek symbols; σ: Staverman reflexion coefficient
The industries include heavy metals, zinc, lead, cadmium, copper, iron, manganese, which is toxic to tissue, organs or cause cancer types [1]. In general, natural water sources contain the amount of organic matter. The fouling of nanofiltration membranes by natural organic matter is due to the physical and chemical interactions [2,3]. The natural organic matter can react with chlorine in the process water treatment and cause formation of disinfection products (DBPs), which is harmful to living organisms and substances have caused impacts on living organisms or cancer in humans [4]. Therefore, membrane separation can be significant in order to remove both organic and inorganic matters from water source.
The efficiency of removal of natural organic matter [5,6] and heavy metal was high using nanofiltration membrane, such as organic matter about 80% [5,7] and removal of heavy metals about 90% [1,8] etc. This research studied the removal efficiency by membrane filtration from the solution, lead metal nanoparticles and organic matter. This study can improve production processes to reduce obstruction and that will affect the performance of the membrane. Including the development of nanofiltration membranes separated by a trial to support the use of a membrane filtration process development and application.
Theory
The flux of solution on the filtration membrane transport of natural organic matter and lead permeate the membrane. As shown in equation1.
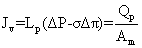 (1)
(1)
The levels of membrane permeate the membrane is measured in Lm-2hr-1kPa-1 is the coefficient of permeability of the membrane. Is measured Lm-2hr-1kPa-1 value ΔP is the average pressure is equal.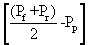 Value is measured in kPa, Osmotic reflection coefficient is approximately equal to the percentage of removal at the surface membrane is the difference in osmotic pressure. Between the surface of the membrane and not part of the Perfect Eight (Osmotic pressure) is measured in kPa is the cross-sectional area of the membrane is measured in m2. Removal efficiency or percentage removal solution (% Rejection,) of nanofiltration membrane system is shown in Equation 2 below.
Value is measured in kPa, Osmotic reflection coefficient is approximately equal to the percentage of removal at the surface membrane is the difference in osmotic pressure. Between the surface of the membrane and not part of the Perfect Eight (Osmotic pressure) is measured in kPa is the cross-sectional area of the membrane is measured in m2. Removal efficiency or percentage removal solution (% Rejection,) of nanofiltration membrane system is shown in Equation 2 below.
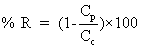 (2)
(2)
Experimental devices
Fouling experiments filtration during this experiment were carried out using an aromatic polyamide thin-film composite ESNA1-4040 membrane, produced by Dow-FilmTec was chosen to determine the efficiency of removal natural organic matter and lead metal solution by nanofiltration performance.
According to the manufacturer, the maximum operating pressure is 600 psi (or 4.16 MPa), maximum feed flow rate is 16 gpm (3.6 m3/hr), membrane active area 85 ft2 (7.9 m2), maximum operating temperature 113°F (45°C) and the operating pH is ranged from 1 to 12.
Flux decline experiments
The experiments were carried out with three liters of solution containing nickel solution (PbCl2) in concentration of 20 mg/L with solution pH of 4 and ionic strengths (0.005, 0.01 and 0.05 M as NaCl). A membrane sheet can be fitted to the cell. The membrane active area is about 41.38 cm2. The Permeate flux was kept in a beaker on the electrical balances.
Extraction and fractionation of NOM
NOM fractions, used in this study, were according to the procedure developed by Leenher and Noyes, 1984; Thurman and Malcolm, 1981 [9,10]. The NOM was filtered on tow filters:
The first is type DH rated the retain 98% of particles 25 μm in diameter and the second filter unit contains filter tube (type AAH) rated to retain 98% of particles 0.3 μm in diameter. The extraction system consisted of two steps. The first one, around 200 liters of water was filtered at pH neutral through the three resin columns of 2 liters (XAD-8, MSC-1 and A-7) connected in series at the rate of 6 liters per hour (Figure 1).
Analysis tools
Analysis of lead can be measured by using Atomic Absorption Spectrophotometer (Perkin Elmer, USA) and for measuring the amount of natural organic matter to use measurements of carbon all by using a TOC-VCPH (Total organic carbon analyzer, model UV mini 1240., Shimadzu Corporation, Japan)
Test membrane
Testing nanofiltration membrane (Bench scale test cell) is shown in Figure 2. To study the obstruction of a solution of lead and compounds starting initial flux is 45 liters per 1, LMH was controlled at a constant 2 h square meter per hour Lm pressure throughout the test. The ability to produce water (percent recovery) was 85% at the flow velocity on the membrane of 0.1 meter per second and time spent is 250 min.
Figure 3 show the effect of NOM and lead ion on solution flux at pH=4 and ionic strength 0.01 N NaCl. The results showed that NOM alone had slightly lower flux decline than those for combined NOM, while lower rejections were found. This indicated less precipitation form in the presence of NOM possibly enhancing metal-NOM interaction presenting in the soluble form and less compacted cake layer at the membrane surface.
Table 1 shows the conductivity and TOC rejection with natural organic matter and lead ion. The lead rejections of combined natural organic matter and metal solution showed a greater rejection than lead ion alone, while solutions in the presence of NOM showed similar average rejection.
| Parameter | Conductivity Rejection (%) | Lead Rejection (%) | TOC Rejection (%) |
|---|---|---|---|
| DI water | 77.2 ± 1.2 | - | - |
| 20 mg/L Pb | 77.3 ± 1.3 | 89.1 ± 1.1 | - |
| 10 mg/L NOM | 78.6 ± 1.3 | - | 96.0 ± 0.8 |
| 20 mg/L Pb+ 10 mg/L NOM | 82.6 ± 1.3 | 93.3 ± 1.1 | 98.3 ± 0.4 |
Table 1: Removal efficiency of nanofiltration membrane.
Effect of the ionic strength
A Case Study of the concentration of natural organic matter and lead solution of 10 and 20 mg/L at pH 4 and the ionic strength was 0.005, 0.01 and 0.05 M. By adding sodium chloride was found that the flux solution of lead with natural organic matter tended to decrease from an increase of salt reduction in flux solution. Joint solution of lead and organic nature of the force charged 0.05 moles per litre, showing a decrease of the flux higher than the ionic strength 0.005 mol/L is shown in Figure 4.
The increase in potency charged with sodium chloride showed that efficiency of removal of salts of membrane potential increases the concentration of the ionic strength capacity of 0.005 to 0.01 moles per litre, compared to the case of salt alone; there were 87.3 ± 1.4 and 82.6 ± 1.3, but the potency charged 0.05 moles per litre, resulting in the removal efficiency decreased, since the capture of salt together with the surface of the membrane increased. Results due to the accumulation layer on the surface of the membrane and pass through the membrane detachment. Efficiency of the indentation filters down to remove the salt removal, Lead and natural organic matter as shown in Table 2.
| Parameter Pb(II) + NOM | Conductivity Rejection (%) | Lead Rejection (%) | TOC Rejection (%) |
|---|---|---|---|
| I.S.=0.005 M NaCl | 87.3 ± 1.4 | 95.3 ± 11 | 93.8 ± 1.3 |
| I.S.= 0.01 M NaCl | 82.6 ± 1.3 | 93.3 ± 1.1 | 98.3 ± 0.4 |
| I.S.= 0.05 M NaCl | 70.4 ± 2.2 | 89.7 ± 0.7 | 90.1 ± 0.7 |
Table 2: Efficiency of rejection of several parameters.
Effect of lead and cadmium
Results from the study of interaction between metals and organic natural matter of the ionic strength with 0.01 mol/l sodium chloride and pH=4 at the concentration of metals, 20 mg per liter, as shown in Figure 5.
Effects of lead and cadmium in the solution to the reduction of flux
From the experimental metal solution concentration of 20 milligrams per liter and the concentration of natural organic matter 10 mg/litre. The potency charged with 0.01 mol/L sodium chloride, pH 4 showed that the flux of the solution is the sum of the natural organic solution may affect the trend of metal. Flux in a manner similar to the decrease of the flux resulting from organic solvents, naturally.
Removal of salt in the form of the conductivity was high due to the charge of the solution binding together, which resulted in the quarantine on the surface of the membrane and increase the efficiency of removal of lead and cadmium were 93.3 ± 1.1% and 93.2 ± 0.7%, respectively, and results in efficient removal of natural organic matter increases as well.
Since the merger between the metal solution and natural organic matter which accumulate on the skin, causing blockages in the system by membrane filtration membrane and the detachment of sifting through the porous membrane was referred to the efficiency of removal of organic matter increased in the case of gold. Red is equal to 98.3 ± 0.4 effective removal of natural organic matter and metal nanoparticles by membrane filtration. As shown in Table 3.
| Parameter | Conductivity Rejection (%) | Metal Rejection (%) | TOC Rejection (%) |
|---|---|---|---|
| Pb (II) | 82.6 ± 1.3 | 93.3 ± 1.1 | 98.3 ± 0.4 |
| Cadmium (II) | 82.8 ± 2.3 | 93.2 ± 0.7 | 84.3 ± 1.9 |
Table 3: Removal efficiency of the membrane from the effects of nanoparticles of metal solution with natural organic matter.
The combination of cationic and anionic lead metal of natural organic matter affecting the obstruction of the nanofiltration membrane. The combined effects of metals and natural organic matter may affect the layer cake (Cake formation) on the surface of the membrane to affect the reduction in the flux of solution and affecting the efficiency of removal by nanofiltration membranes. The increases of the features of the solution affect the efficiency and remove blockages. Since the capture of metals and organic compounds of natural causes blockage in the hole and / or accumulated on the surface of the membrane.