
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495
+44 1478 350008

ISSN: 2161-0495
+44 1478 350008
Commentary - (2021)
There is a defined, but poorly described, relationship between Trastuzumab and Cutaneous Lupus Erythematosus, and whether Trastuzumab causes CLE or simply leads to dermatologic manifestations of latent Lupus Erythematosus has yet to be established. The link between the two seems now more relevant than ever, as Trastuzumab has recently been theorized as a possible therapy for Lupus Nephritis. As such, any cutaneous rash or skin lesion that appears after the administration of Trastuzumab should lead to suspicion of Cutaneous Lupus Erythematosus. We report a case of Cutaneous Lupus Erythematosus, proven by both skin biopsy and serology, in a female patient on her 9th cycle of Trastuzumab emtasine for breast cancer.
Trastuzumab; Subacute cutaneous lupus; Lupus nephritis
Cutaneous Lupus Erythematosus (CLE), has been previously linked with the treatment of HER2+ breast carcinoma due to aromatase inhibitors, but rarely with the use of Trastuzumab. In the cases were it was admitted that Trastuzumab led to CLE (and not simply to rashes) the diagnosis was always supported by the presence of an annular pattern of eruption and positive testing for Anti-Ro/SSA [1-4].
A 77-year-old female patient with HER2+ breast adenocarcinoma presented with a generalized rash and pruritus after the 9th cycle of Trastuzumab emtasine (the only medication that the patient was under). The lesions presented as erythematous plaques, some with desquamative properties, mainly on the lateral side of the upper right arm, on the upper thoracic region dorsum (with a predominance of desquamative plaques in the interscapular area), malar regions and forehead (Figures 1).
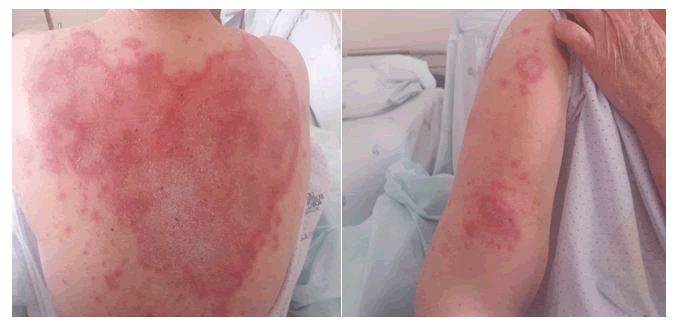
Figure 1: Rashes identified after the 9th cycle of Trastuzumab Emtasine.
A skin biopsy revealed CLE, associated with positive antinuclear antibodies (speckled pattern, 1:320) and positive Anti-SSA (3+), in a patient that was previously ANA negative (with no previous Anti-SSA test). She was admitted to the Internal Medicine Ward, and throughout her stay she never showed any signs of fever, respiratory or urinary symptoms, arthralgia or synovitis. She started a short course of Prednisolone (40 mg daily, tapered off the following days) plus Hydroxicloroquine (400 mg daily).
There was complete remission of the skin lesions after two weeks. Both prednisolone and hydroxicloroquine were stopped, with no relapse of the lesions. The patient did not restart Trastuzumab emtasine.
Our case seems to once again support the link between the Trastuzumab and CLE, as it clearly shows a compatible dermatologic pattern, positive skin biopsy and positive serology for Lupus, in a patient whose only related external factor (environmental or iatrogenic) was the use of Trastuzumab emtasine.
Altough the link between these lesions and trastuzumab seems undeniable, the fact that they only originated after the 9th cycle, and that the description of Trastuzumab-associated CLE seems so rare in the literature, seems to suggest that either CLE is induced by the cumulative dosage of this drug in susceptible patients, or that this is a rare and idiosyncratic drug reaction.
Systemic manifestations also seem to be absent in both our case and in previously described cases, leading us to question whether Trastuzumab causes true CLE or simply causes Lupuslike rashes[4-7].
We would also like to note that it has also been previously established that HER2+ levels increase during Lupus flares, with recent proposals of studying the use of Trastuzumab as a possible therapy for Type III and IV Lupus Nephritis.
Any such studies must account for the possibility of a paradoxical dermatologic Lupus flare, as described in our case.
Citation: Teixeira JA, Catarina C, Ines S, Claudia J (2021) Subacute Cutaneous Lupus Secondary to Trastuzumab Emtasine. J Clin Toxicol. S19:005.
Received: 13-Sep-2021 Accepted: 27-Sep-2021 Published: 04-Oct-2021 , DOI: 10.35248/2161-0495.21.s19.005
Copyright: © 2021 Teixeira JA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.