
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2023)Volume 13, Issue 5
Background: Novel medications for treating any SARS-CoV-2 variant and similar pandemic viruses are sought. Growing evidence connects coronavirus’ binding, fusion, and replication, as well as evolved acute pulmonary infections, with locally induced acidity. Stabilized Amorphous Calcium Carbonate (ACC) has demonstrated preclinical and clinical efficacies for treating biomedical conditions associated with pH modulation effects by delivering alkaline carbonate content to acidified environments.
Objectives: The study aimed to establish the safety, tolerability, and efficacy of ACC, named AMOR-18 (manufactured by Amorphical LTD), for treating hospitalized patients with moderate-to-severe SARS-CoV-2, administered as a combination of sublingual powder and inhaled suspensions alongside the Best Available Treatment (BAT).
Methods: A Phase 1/2 trial-a phase 1 (open label single arm study) expanded into a phase 2, multicenter, prospective, 1:1 randomized, double-blind, placebo-controlled, concomitantly with Best Available Treatment (BAT) was performed on hospitalized, moderate-to-severe COVID-19 patients. Patients in the active arm received four daily sublingually administered doses of 1,475 mg ACC powder and three inhaled doses of 370 mg ACC in 10 ml suspensions (total daily doses of 5.900 mg in the form of powder and 1,110 mg as a suspension of ACC). The intended primary efficacy outcomes were patient improvement rate, defined as a reduction of at least one point in an established eight-category Disease Ordinal Scale (DOS), used in COVID-19 clinical trials; statistically significant reducing time from treatment to discharge; and statistically significant prevention of patient transfer to the Intensive Care Unit (ICU) and death.
Results: After a successful safety study with six patients in Stage 1, the double-blind study was performed on sixty patients that were equally randomized (30/30) to the active and placebo arms with similar DOS severity. The most significant outcome was the prevention of ICU transfer and death (0%) in the active arm compared to seven ICU transfers and three deaths in the placebo arm (23%; Fisher’s P=0.011). The patient improvement rate was significantly higher in the ACC (93%; 90% CI=82%-98%) compared to the placebo arm (73%; 90% CI=59%-84%) in the intention-to-treat sets. All patients in the active arm were discharged within 10 days from treatment initiation, and only one related adverse effect (constipation) was reported. There were no significant differences in responses by age, gender, comorbidities, and vaccination status.
Conclusion: This early clinical study demonstrates a clinically meaningful effect in treating moderate-to- severe COVID-19 patients with a combination of sublingually and inhaled doses of ACC, primarily preventing disease deterioration and death, as well as enhancing improvement and recovery rates.
COVID-19; Amorphous calcium carbonate; Clinical study; Acidosis; Anti-inflammatory; Randomized double-blind clinical trial; Sublingual; Inhalation
Messenger-RNA (mRNA) vaccines against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the causative agent of the Coronavirus Disease 2019 (COVID-19), have demonstrated great success in preventing severe disease and death. However, growing evidence suggests that SARS-CoV-2 variants can escape or disrupt the immune response induced by existing vaccines [1-4]. The currently available vaccines are less effective against new variants of the disease and, perhaps, future viral pandemics of new strains of coronaviruses associated with acidotic progression. Therefore, new effective and innovative antiviral and anti-inflammatory therapies are still warranted. Additional treatment approaches are under development or already approved and continuously monitored by the WHO [5]. There is an urgent need to develop safe drugs that are effective in treating all the stages of the disease and new emerging coronavirus stains.
A growing number of fundamental studies report that local extracellular low pH is often associated with severe forms of COVID-19 and high lactate levels, indicating a progressive hypoxia stage [6-10]. Dysregulations or impairments of the innate and adaptive immune systems may participate in tissue damage and exacerbate disease severity at lower pH levels [11,12].
Both hypoxia and inflammation contribute to excessive local acidification in COVID-19 and vice versa [13]. In addition, preexisting or progressive local acidity was reported to accelerate viral activities at early infection stages of various SARS-CoVs, including increased expression of the Angiotensin-Converting Enzyme-2 (ACE-2) receptors, better binding and fusion of the virus into cells, and accelerated replication [13]. Recent studies suggest that the conformation of the SARS-CoV-2 S-protein can be sensitive to changes in pH [14]. Thus, treatments that locally target and modulate low pH may become efficient approaches for a range of disease severity from mild to critical.
Before the COVID-19 pandemic, Amorphous Calcium Carbonate (ACC) had been studied in other early-stage clinical trials and consistently proved to be safe and potentially effective in treating various diseases, including calcium deficiencies (hypoparathyroidism) and late-stage cancers (A Study Comparing Amorphous Calcium Carbonate (ACC) versus Crystalline Calcium (CCS) in Hypoparathyroidism Patients (AMCS009) [15,16]. ACC has demonstrated double absorption of calcium and much higher solubility in the body’s pH range compared to Crystalline Calcium Carbonate (CCC) forms [17]. Due to its alkaline carbonate content, ACC is postulated to modulate acidic pH around cells and organs that results from inflammation and numerous diseases and body stressors [18]. The primary particles of ACC are nanometric, unlike CCC. These nanometric particles can penetrate through mucous membranes into circulation without the need to dissolve the calcium carbonate molecular structure and decompose their alkaline carbonate load into carbon dioxide, which usually occurs in the stomach during oral consumption of calcium supplements and antacid relief products. The released carbonate ions from the ACC at pH ranges slightly below the normal (7.35 to 7.45) immediately eliminate acidic protons (H+) and convert them to bicarbonate ions. Bicarbonate ions are the main natural pH regulators and critical electrolyte balancing agents in the body and continue the pH modulation process.
Objectives, patients, and study management
This study aimed to assess the safety, tolerability, and efficacy of Amorphous Calcium Carbonate (ACC, named AMOR-18, manufactured by Amorphical LTD, Nes Ziona, Israel), administered as a combination of sublingual powder and suspension inhalation alongside the Best Available Treatment (BAT), for the treatment of moderate-to-severe COVID-19 in hospitalized patients. A single primary endpoint was set and defined as the improvements in the severity of disease signs and symptoms, assessed by an eight-category Disease Ordinal Scale (DOS), adapted from previous COVID-19 studies. A secondary efficacy outcome was the prevention of critical deterioration, assessed by the transferring of patients to Intensive Care Units (ICU) with or without invasive mechanical ventilation or Extracorporeal Membrane Oxygenation (ECMO).
Due to high concerns for the safety of medical staff by the spread of virus-contaminated aerosol, the inhalation procedure was carefully managed by using a designated disposable inhalation device connected to the hospital’s air or oxygen supply. The device delivers the drug aerosols in a closed and filtered system with no aerosol escape to the environment (FDA approved, Circulaire® II, manufactured by Westmed Inc, Tucson, Arizona, USA). In other inhalation practices, any efficient suspension inhaler, including ones designated for home use, would be appropriate.
A preliminary training phase was performed as a single open-label clinical study, assessing the tolerability and safety of ACC and the method of product administration. Six patients were recruited in three research hospitals in Israel. All six patients recovered and were released from the hospitals within a few days without any drug-related adverse events.
Following data assessment and recommendation by an independent Data Safety Monitoring Board (DSMB) to continue the study, a Phase 2 trial was established as a prospective, multicenter, randomized, double-blind, placebo-controlled trial. The enrollment target for the initial Phase 2 assessment was 100 subjects with randomization of 1:1. The active arm received sublingual and inhaled ACC alongside the BAT, while the control arm received a placebo and the BAT. The phase 2 trial was performed in three university hospitals in Israel (Ziv, Shamir, and Kaplan), following approvals by the ethics committees at each site. Each hospital independently entered the study after completing its board review, staff assignments, and training. It should be noted that due to the various modes of operations of the different centers during the pandemic, participating staff and medical supervisors of the designated COVID-19 departments were frequently rotated for spreading the heavy burden of treating the isolated patients. All eligible patients were required to sign enrollment consent before entering the study.
Study design, procedure, efficacy, and safety endpoints
Table 1 summarizes the study design, inclusion and exclusion criteria, daily dosage, drug modes and frequency of administration, efficacy endpoints, and methods of patient progress assessment. The primary endpoint was the improvement rate, defined as at least a one-point improvement in the DOS score [19]. Subjects transferred to the ICU or who died was considered to have scores of 7 and 8, respectively. Meanwhile, those discharged from the hospital scored 1 or 2. The secondary efficacy endpoints were the duration of treatment until discharge, transfer to ICU, or death.
| Overall study design | |
|---|---|
| Prospective, multicenter, 1:1 randomized, double-blind, placebo-controlled trial of 100 subjects, hospitalized with moderate-to-severe conditions. Patients were randomized to active + Best Available Treatment (BAT) or placebo + BAT. | |
| Patient enrollment distribution: 61.7% (37/60) at Ziv, 31.7% (19/60) at Shamir Medical Center, and 7.7% (4/60) at Kaplan. | |
| Inclusion criteria | Exclusion criteria |
| Males and females of age between 18 to 80 years diagnosed with SARS-CoV-2 and hospitalized. | Pregnant or breastfeeding females. |
| Having imaging evidence for lung involvement. | Patients with non-SARS-CoV-2 related pneumonia or pulmonary disease, tracheostomy, or mechanical ventilation. |
| With or without supplemental oxygen at enrollment. A screening Ordinal Disease Severity Score (DOS) of 3 to 6. | Hypercalcemia (>11.0 mg/dL). |
| Hyperphosphatemia (>4.5 mg/dL). | |
| Participation in another study. | |
| ACC administration procedure | |
| Three inhaled suspensions and four doses of sublingual AMOR-18 powder formulation per day (two hermetically sealed packets each time). | |
| Total daily sublingual dosage: 5900 mg of powder (2000 mg Ca: 2400 mg carbonate. | |
| The inhaled active suspension was prepared before each administration, using a double-pack kit, instantaneously forming 10 ml of ACC suspension; 45 mg Ca and 69 mg carbonate per inhalation (135 and 207 mg, respectively daily dose). | |
| The placebo sublingual powder: mainly microcrystalline methylcellulose powder at the same particle size range, weight, color, and flavor as the investigational product. | |
| The inhaled placebo: two tubes of saline (same volume as the active suspension). | |
| Efficacy endpoints | Methods of assessment |
| Primary end points | Eight-category ordinal scale |
| Change in severity rating of disease using an eight-category ordinal scale measured on days 7, 14, and 21. Changes were measured as improvements > 1 point from the baseline score. | (Adopted from a the Remdesivir study registration; ClinicalTrials.gov Identifier: NCT04280705; Feb 21, 2020) |
| Recovery rate: Defined as scores < 3 using the eight-category ordinal scale. | Not hospitalized and no limitations of activities. |
| Not hospitalized, with limited activities, home oxygen requirement, or both. | |
| Hospitalized, not requiring supplemental oxygen, and no longer requiring ongoing medical care (used if hospitalization was extended for infection-control or other nonmedical reasons). | |
| Hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (related to SARS-CoV-2 or other medical conditions). | |
| Hospitalized, requiring any supplemental oxygen. | |
| Hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices. | |
| Hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation. | |
| Death. | |
| Secondary end points | Assessment methods |
| Duration of hospital stay. | Percentage of patients with scores < 3 on the eight-category ordinal severity scale. |
| Duration of Intensive Care Unit (ICU) stay. | Daily probability of staying at the hospital. |
| Duration of mechanical ventilation use (if needed). | Percentage of patients transferred to the ICU. |
| Duration of oxygen supplementation. | Percentage of patients who died. |
| Duration of no oxygen use. | |
| Safety end points | Assessment methods |
| Frequency and severity of adverse events related to the study drug. | Count and percentage of patients with hypercalcemia per ACC dose and percentage of patients with hypercalciuria (Urine and blood samples collected on days 4, 7, 14, and 21). |
ACC = Amorphous calcium carbonate; SARS-CoV-2 = Severe Acute Respiratory Syndrome Coronavirus Virus
Table 1: The study design parameters. The table includes efficacy outcome measures and their methods of evaluation, overall participation in each center, the mode of administration, and daily dosage and their mode of preparation.
The inclusion criteria were participants between 18 years to 80 years old, diagnosed with SARS-CoV-2 and hospitalized with imaging evidence for lung involvement, with or without supplemental oxygen at enrollment (Table 1). All participants had a screening DOS score of 4 to 6, which required their hospitalization, associated with at least 1 BAT in most cases. Exclusion criteria were pregnant or breastfeeding women, patients with non-SARS-CoV-2-related pneumonia or pulmonary disease, tracheostomy or mechanical ventilation, hypercalcemia (>11.0 mg/dL), hyperphosphatemia (>4.5 mg/dL), or patients participating in another study.
Each patient was treated according to their clinical condition and at the discretion of the attending physician with various available BATs at the time of admission, including remdesivir, dexamethasone, flixotide (fluticasone propionate) and aerovent. The designed study period per patient was set to a maximum of 22 days, or until the patient was discharged from the hospital, transferred to ICU, or died. Nevertheless, none of the participants remained in the study for more than 12 days.
Table 1 also includes the overall participation in each hospital, reflecting the time at which each center joined the study and passed the introduction and training period of the medical staff. In addition, the table describes the treatment procedure and dosage per patient receiving either the active or the placebo arm.
Procedures
The full description of study medical treatment and procedures are provided in the protocol section of the Supplementary document. The ACC administration consisted of three inhaled suspensions and four doses of sublingual powder formulation per day (two hermetically sealed packets each time; total of eight packets per day). The total daily sublingual dosage of the active substance was 5900 mg of powder weight containing 2000 mg calcium and 2400 mg carbonate. The inhaled active suspension was freshly prepared by rapidly transferring one solution from a small vial into a larger vial of a second solution, sealing the larger vial and shaking it vigorously, instantaneously forming a 10 ml of ACC suspension consisting of 45 mg calcium and 69 mg carbonate (135 mg and 207 mg, total daily doses respectively). The prepared solution was administered using a double-pack kit.
The placebo sublingual powder formulation consisted of mainly microcrystalline methylcellulose powder at the same particle size range, weight, color, and flavor as the investigational product powder. The placebo inhalation kit contained two tubes of saline of the same volumes as the inhaled investigational product. A special standardized inhaler was used, suitable for safe hospital use during the pandemic with no risk of spreading contaminated aerosol. The information about the inhaler is found in the protocol provided in supplementary of this article.
Data collection
The study data was collected using CASTOR's Electronic Data Capture (EDC) by the different participating sites, based on electronic Case Report Forms (CRFs), generated by an external qualified Data Management subcontractor. The individual data of each participating patient was collected by the participating investigators according to the protocol study design and table of activities. The data was documented either in the site’s electronic file of the patient (Source document) by the medical provider, or directly into the eCRF as outlined by the protocol. Clinical data from the patient/participant file/source was transferred by the study coordinator into the participant eCRF.
The data were solely and objectively produced by the independent hospital investigators, reflecting the patients’ status during the course of the study. The sponsor or his CRO designer reviewed or monitored the data only for their completeness and consistency and issued a query for the investigators when incomplete data was recognized by the system.
Statistical analysis
Statistical analyses were independently performed by Biostats, Statistical Consulting LTD, using SAS® v9.4 (SAS Institute, Cary, NC, USA). Baseline demographics, other characteristics, and safety analyses were performed on all randomized subjects. The required significance level of findings was selected at 10% as this is an underpowered Phases 1 and 2 studies consisting of a small number of participants. All statistical tests were two-tailed, with corresponding 90% confidence intervals. Demographics and baseline data were compared between the study arms with t-test (continuous variables) or Fisher’s exact test (categorical variables). The proportions of subjects achieving an improvement of at least one point on the DOS at the discharge/end of study visit are presented along with two-sided 90% Wilson’ score Confidence Interval (CI) and compared between the treatment arms with a Fisher’s exact test. Subgroups were analyzed by age group, sex and vaccination status is presented. The Mantel-Haenszel test was used to compare the study arms, adjusted for the subgroup parameter (either age group, sex, or vaccination status). The Breslow-Day test was used to test the homogeneity of the treatment effect across the subgroups.
Recovery rates are presented along with two-sided 90% Wilson’ scores Confidence Interval (CI) and compared between the treatment arms with a Fisher’s exact test. Death and/or transfer to ICU rates are presented along with two-sided 90% Wilson’ score Confidence Interval (CI) and compared between the treatment arms with a Fisher’s exact test. Subgroup analyses of the death/ICU rates by age group, sex and vaccination status are presented. The Mantel-Haenszel test was used to compare the study arms, adjusted for the subgroup parameter (either age group, sex, or vaccination status); and logistic regression (instead the Breslow-Day test, since it was not appropriate due to the sparsity of the data).
The Supplementary for this article includes additional details regarding the statistical analysis procedures and tables.
Patients
The study began in January 2021, was locked in March 2022, and was performed throughout two major pandemic waves (Delta and Omicron) in Israel. The vast majority of the study subjects (except 3 patients) have participated during the Delta waive. All the three assumed Omicron patients recovered well and none of them was admitted to ICU. The enrolment, randomization, demographics, and vaccine status of the study participants are illustrated in Figure 1. Sixty eligible patients were enrolled at three medical centers, and 30 were randomized to each ACC and placebo arm. Only minor protocol deviations were observed. Therefore, all the reported results are based on the Intent-To-Treat (ITT) analysis.
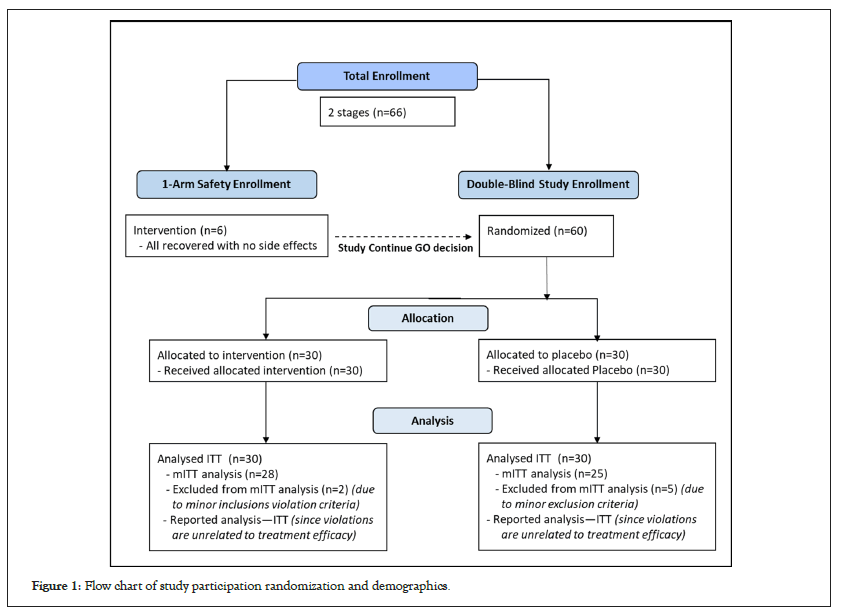
Figure 1: Flow chart of study participation randomization and demographics.
Table 2 shows the baseline characteristics of patients in both arms. The mean age of all participants was 53.9 years, and 68.3% were males. Almost 70% of the subjects were not vaccinated, and only about 20% had received two or three doses of a SARS-CoV-2 vaccine.
| Characteristic | Active | Placebo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accountability | N | % | N | % | Total | ||||||||
| ITT analysis set | 30 | 100.0% | 30 | 100.0% | 60 | ||||||||
| Gender | N | % | N | % | P-value* | ||||||||
| Female | 14 | 46.67% | 5 | 16.67% | 0.025 | ||||||||
| Male | 16 | 53.33% | 25 | 83.33% | |||||||||
| Ethnicity | N | % | N | % | P-value* | ||||||||
| Not hispanic/Latino† | 29 | 69.67% | 25 | 83.33% | 0.19 | ||||||||
| Unknown | 1 | 3.33% | 5 | 16.67% | |||||||||
| Age (years) | N | Mean | SD | Min | Med | Max | N | Mean | SD | Min | Med | Max | P-value** |
| 30 | 49.9 | 8.9 | 28.7 | 48.3 | 64.9 | 30 | 58 | 11.8 | 28.2 | 58.5 | 80.6 | 0.0037 | |
| Vaccination status | N | % | N | % | P-value* | ||||||||
| Not vaccinated/ unknown | 22 | 73.33% | 21 | 70.00% | 1.00 | ||||||||
| Vaccinated | 8 | 26.67% | 9 | 30.00% | |||||||||
| Severity at screening | N | % | N | % | P-value* | ||||||||
| Moderate | 9 | 30.00% | 8 | 26.67% | 1.00 | ||||||||
| Severe | 15 | 50.00% | 16 | 53.33% | |||||||||
| Unknown | 6 | 20.00% | 6 | 20.00% | |||||||||
Note: ITT=Intention-to-treat
† All subjects were from Europe, Middle East, North Africa, and Ethiopia
* Fisher’s exact test
** T-test
Table 2: Accumulated patient characteristics during the initial screening stage.
A significantly higher proportion of females were randomized to the ACC arm compared to the placebo arm (47% versus 17%, p=0.025). Similarly, the mean age of the active arm was significantly lower than in the placebo arm (49.9 versus 58.0, p=0.0037). These two important parameters led to an additional statistical covariance analysis, which validated that these differences did not affect the outcome results. This imbalance between the two arms occurred by chance (the randomization was stratified by site, but not by sex or age group) with no statistical explanation, except the fact that the overall number of participants was relatively low. This was an exploratory and underpowered study. The protocol called for 100 patients, which is still a low number of subjects, but the various medical centers have been able to recruit only 60 patients due to the high constraints during the peaks of the pandemic waves. The low recruiting rates are reflected in the discussion section.
There were no significant differences between the two treatment arms regarding disease severity, vitals and symptoms at screening, vaccination status, and BAT administration (Table 3).
| Symptoms, vitals, and BAT at screening | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC arm | Placebo arm | ||||||||||||
| Baseline Respiratory, SpO2, Temperature | N | Mean | SD | Min | Med | Max | N | Mean | SD | Min | Med | Max | P-value* |
| Respiratory Rate SpO2 | 12 | 20.5 | 5 | 12 | 21 | 30 | 8 | 22.4 | 6.2 | 11 | 23.5 | 30 | 0.46 |
| Without O2 supply | 14 | 94.7 | 3.9 | 86 | 95.5 | 99 | 12 | 95 | 1.5 | 92 | 95.5 | 97 | 0.8 |
| With O2 supply | 14 | 96.2 | 2.5 | 93 | 96 | 100 | 16 | 94.9 | 2.5 | 90 | 95.5 | 99 | 0.17 |
| O2 supply unknown | 1 | 98 | . | 98 | 98 | 98 | . | . | . | . | . | . | |
| Temperature (°C) | 28 | 36.8 | 0.5 | 36 | 36.8 | 38.8 | 28 | 36.8 | 0.5 | 36 | 36.8 | 38.2 | 0.98 |
| Common symptoms | N | % | N | % | P-value** | ||||||||
| Aches and pains | 16 | 53.33% | 22 | 73.33% | 0.18 | ||||||||
| Cough | 28 | 93.33% | 27 | 90.00% | 1 | ||||||||
| Diarrhea | 7 | 23.33% | 5 | 16.67% | 0.75 | ||||||||
| Dyspnea | 22 | 73.33% | 19 | 63.33% | 0.58 | ||||||||
| Fever | 14 | 46.67% | 16 | 53.33% | 0.8 | ||||||||
| Headache | 4 | 13.33% | 5 | 16.67% | 1 | ||||||||
| Loss of taste/smell | 5 | 16.67% | 8 | 26.67% | 0.53 | ||||||||
| Loss of appetite | 3 | 10.00% | 1 | 3.33% | 0.61 | ||||||||
| Nausea | 1 | 3.33% | 5 | 16.67% | 0.19 | ||||||||
| Sore throat | 6 | 20.00% | 4 | 13.33% | 0.73 | ||||||||
| Tachycardia | 2 | 6.67% | 3 | 10.00% | 1 | ||||||||
| Tiredness | 24 | 6.67% | 22 | 73.33% | 0.76 | ||||||||
| Vomiting | 2 | 100.0% | 4 | 13.33% | 0.67 | ||||||||
| Tachycardia | 2 | 6.67% | 3 | 10.00% | 1 | ||||||||
| Additional BAT | N | % | N | % | P-value** | ||||||||
| At least 1 treatment | 27 | 90.00% | 28 | 93.33% | 1 | ||||||||
| Aerovent | 9 | 30.00% | 11 | 36.67% | 0.78 | ||||||||
| Dexamethasone | 15 | 50.00% | 15 | 50.00% | 1 | ||||||||
| Flixotide | 8 | 26.67% | 11 | 36.67% | 0.58 | ||||||||
| Remdesivir | 23 | 76.67% | 21 | 70.00% | 0.77 | ||||||||
| Vitamin D3 | 16 | 53.33% | 19 | 63.33% | 0.6 | ||||||||
Note: ACC=Amorphous calcium carbonate
* T-test
** Fisher’s exact test
Table 3: Patient symptoms and Best Available Treatment (BAT) at the screening stage (before ACC administration).
Primary outcome
The study consisted of one Primary Endpoint (PEP) the improvement rate, defined as at least a one-point improvement in the DOS score. Although the PEP was evaluated several times during the course of the study, only the last measurement was counted for the statistical evaluation (a) prior to release from the hospital (i.e., the recovery point) or (b) transfer to ICU. Yet, measuring the scoring during the hospitalization was very important for the practical evaluation of the patients from a medical standpoint as well as giving a better insight to the trends of the improvement rates between the two arms.
Table 4 comprehensively summarizes the DOS ranking and the overall improvement and recovery rate analyses. At screening, all subjects had a DOS score between 4 and 6, with 77% of the participants having scores of 5-6. The scoring for each of the treatment groups was comparable, and there was no statistically significant difference between the two study arms (P=0.67). The average DOS score at screening was almost identical for the active (4.7) and placebo arms (4.8). This is a partial indication for the non-influential effects of the sex and age imbalanced numbers of participants between the arms.
| Eight-point ordinal scale analysis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At screening | Eight-point ordinal scale | |||||||||||||||
| 4 | 5 | 6 | Total | P-value* | ||||||||||||
| N | % | N | % | N | % | N | % | 0.68 | ||||||||
| ACC | 11 | 37 | 18 | 60 | 1 | 3 | 30 | 100 | ||||||||
| Placebo | 8 | 27 | 20 | 67 | 2 | 7 | 30 | 100 | ||||||||
| Average Ordinal Score (DOS) by day of treatment | ||||||||||||||||
| Day | Screening | 7 | 10 | 14 | Discharge | |||||||||||
| N | OS | N | DOS | N | DOS | N | DOS | N | DOS | |||||||
| ACC | 30 | 4.7 | 30 | 2.4 | 30 | 1.8 | 30 | 1.6 | 27 | 2 | ||||||
| Placebo | 30 | 4.8 | 30 | 3 | 30 | 2.8 | 30 | 2.8 | 29 | 2.4 | ||||||
| Overall ordinal scale improvement by at least one point | ||||||||||||||||
| N | n | % | 90% Wilson score CI | P-value* | ||||||||||||
| ACC | 30 | 28 | 93 | (82%;98%) | 0.08 | |||||||||||
| Placebo | 30 | 22 | 73 | (59%;84%) | ||||||||||||
| One-point ordinal scale improvement by gender | P-value† | P-value†† | ||||||||||||||
| Female | ACC | 14 | 13 | 93 | 0.034 | 0.73 | ||||||||||
| Placebo | 5 | 3 | 60 | |||||||||||||
| Male | ACC | 16 | 15 | 93 | ||||||||||||
| Placebo | 25 | 19 | 76 | |||||||||||||
| One-point ordinal scale improvement by age (yeas) | P-value† | P-value†† | ||||||||||||||
| Up to 55 | ACC | 21 | 19 | 90 | 0.06 | 0.21 | ||||||||||
| Placebo | 11 | 9 | 82 | |||||||||||||
| Over 55 | ACC | 9 | 9 | 100 | ||||||||||||
| Placebo | 19 | 13 | 68 | |||||||||||||
| One-point ordinal scale improvement by vaccination status | P-value† | P-value†† | ||||||||||||||
| 0.038 | 0.47 | |||||||||||||||
| Not vaccinated/ unknown | ACC | 22 | 20 | 91 | ||||||||||||
| Placebo | 21 | 15 | 71 | |||||||||||||
| Vaccinated | ACC | 8 | 8 | 100 | ||||||||||||
| Placebo | 9 | 7 | 77 | |||||||||||||
| Recovery rates | ||||||||||||||||
| N | n | % | 90% Wilson score CI | P-value* | ||||||||||||
| ACC | 30 | 30 | 100 | (92%;100%) | 0.011 | |||||||||||
| Placebo | 30 | 23 | 77 | (62%;87%) | ||||||||||||
Note: ACC = Amorphous calcium carbonate;
*Fisher’s exact test; †Mantel-Haenszel Test; ††Breslow-Day Test
Table 4: Improvement and recovery analyses based on the eight-category ordinal scale and recovery rates.
The Figures 2A and 2B illustrates the daily improvements in mean DOS for the two arms. For clarity, the DOS scores were slightly grouped into 5 scoring categories: 1-2 (mild), 3-4 (moderate), 5-6 (severe), 7 (critical, ICU), and 8 (death). Figure 3 emphasizes the improvement rates by comparing the DOS scores of admitted and isolated COVID-19 patients, with (a) patients recovering or (b) deteriorating into critical states. The improvement rate was statistically significantly higher in subjects randomized to the ACC arm compared to the placebo arm (Fisher’s P=0.080).
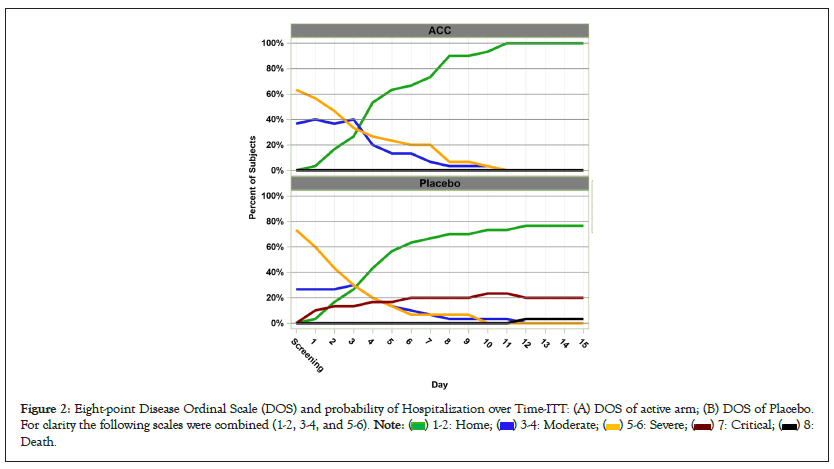
Figure 2: Eight-point Disease Ordinal Scale (DOS) and probability of Hospitalization over Time-ITT: (A) DOS of active arm; (B) DOS of Placebo.
For clarity the following scales were combined (1-2, 3-4, and 5-6). 
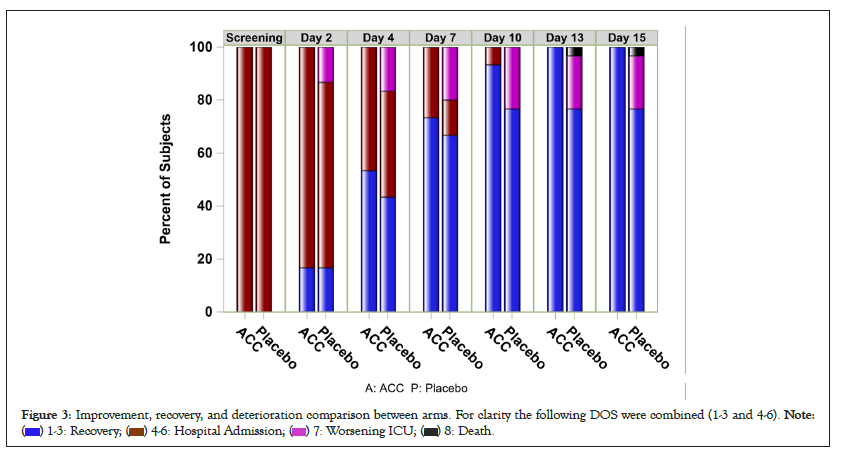
Figure 3: Improvement, recovery, and deterioration comparison between arms. For clarity the following DOS were combined (1-3 and 4-6). 
The average improvement rates from the screening at selected intervals (Days 7 and 14), and discharge days are displayed in Table 4. Subjects transferred to the ICU during the study were considered as treatment failures. Both arm groups continue to show improvements in DOS scores. However, the curves for the two arms diverge from Day 7 onwards with a clear advantage for the active treatment. After day 10, the improvement delta between the arms became higher than 1.0 on Day 10 (active arm=1.8 vs. placebo=2.8) and even higher at Day 14 (1.6 vs. 2.8).
The improvement rates were analyzed by the covariance of gender, age, and vaccination status. The rates for all the subcategories were significantly greater in subjects in the active than those in the placebo arm (Mantel-Haenszel Test P=0.05). The Breslow-Day test for homogeneity was added to assess the similarity of ACC statistical effect by age, gender, and vaccination status. The ACC efficacy was similar across all subgroups. Table 4 compares the statistical results of these subgroups as reflected between the scoring of the 2 arms. The ACC effect size were found similar in male and female subjects, younger and older subjects, and vaccinated or not vaccinated subjects as indicated by Breslow-Day test p-values, compared the significant improvement rates for the ACC arms in each of the subcategories as indicated by the Mantel-Haenszel Test.
Secondary outcome
Preventing disease worsening and mortality has become the main outcome of determining the efficacy of vaccines and other new treatments for SARS-CoV-2 [5]. Hence, it was imperative to assess these aspects in this study. Preventing disease deterioration in COVID-19 patients is considered the most critical outcome of current treatments, including the major effect achieved by mass vaccination.
The recovery rate was assessed by subjects having DOS scores ≤ 3 on discharge. Subjects admitted to the ICU or who died during the study was considered as “not recovered”, regardless of their final score. The recovery rate was statistically significantly higher in the active than the placebo arm (100% vs. 76%, P=0.011). No patient in the active arm was transferred to ICU or died, while seven subjects in the placebo arm were admitted to the ICU, and three died while receiving the BAT (Table 5). The recovery rates are also illustrated in Figure 4 in the form Kaplan-Meier hospitalization probability curves as a function of days from the first dose to discharge from the hospital.
| Death and ICU hospitalization | ||||||
|---|---|---|---|---|---|---|
| Deterioration | N | n | % | 90% Wilson score CI | P-value* | |
| ICU | ACC | 30 | 0 | 0 | (0%;8%) | 0.011 |
| Placebo | 30 | 7 | 23 | (13%;38%) | ||
| Death | ACC | 30 | 0 | 0 | (0%;8%) | 0.24 |
| Placebo | 30 | 3 | 10 | (4%;23%) | ||
| Death or ICU hospitalization | ||||||
| Gender | P-value (Mantel-Haenszel Test) | P-value (Type 3, Logistic Regression) | ||||
| Female | ACC | 14 | 0 | 0 | 0.01 | 0.85 |
| Placebo | 5 | 1 | 20 | |||
| Male | ACC | 16 | 0 | 0 | ||
| Placebo | 25 | 6 | 24 | |||
| Age (years) | P-value (Mantel-Haenszel Test) | P-value (Type 3, Logistic regression) | ||||
| Up to 55 | ACC | 21 | 0 | 0 | 0.013 | 0.61 |
| Placebo | 11 | 2 | 18 | |||
| Over 55 | ACC | 9 | 0 | 0 | ||
| Placebo | 19 | 5 | 26 | |||
| Vaccination status | P-value (Mantel-Haenszel Test) | P-value (Type 3, Logistic regression) | ||||
| Not vaccinated/ unknown | ACC | 22 | 0 | 0 | 0.0056 | 0.93 |
| Placebo | 21 | 5 | 23 | |||
| Vaccinated | ACC | 8 | 0 | 0 | ||
| Placebo | 9 | 2 | 22 | |||
Note: *Fisher’s exact test;
ACC=Amorphous calcium carbonate; ICU=Intensive Care Unit
Table 5: Deterioration of patients indicated by transferring to ICU and death rates.
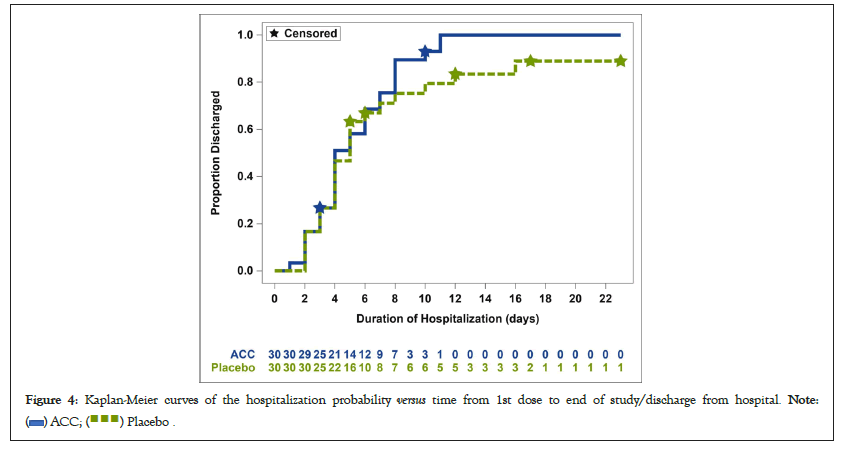
Figure 4: Kaplan-Meier curves of the hospitalization probability versus time from 1st dose to end of study/discharge from hospital. 
The combined ICU and death rates were also analyzed by sex, age, and vaccination status to reassure no covariant effects. The combined ICU and death rates across subgroups were statistically significantly lower in subjects randomized to the ACC compared with the placebo arm (Mantel-Haenszel test P-values of 0.010, 0.013, and 0.0056, respectively; Table 5). The ACC effect size was similar in males, younger, and vaccinated participants compared to females, older, and non-vaccinated subjects as validated by the Logistic Regression Type III P-values as manifested in Table 5.
Subjects admitted into ICU while on placebo treatment had higher rates of comorbidities including heart failure, obesity, diabetes, asthma and hypertension. A full list and details about subjects and their comorbidities are provided in the statistical analysis in the Supplementary Appendix. There was insufficient data to define comorbidity trends that specifically characterize the group transferred to ICU out of the rest of the placebo arm subjects.
Duration of hospitalization was initially defined as a secondary endpoint. Since numerous patients were offered the treatment after several days of hospitalization, with no relief or further deterioration (with BATs), this outcome was redefined as the time from the first dose to discharge (instead of admission-to-discharge period). The duration of hospitalization was not significantly different between the study arms (log-rank test P=0.29), as illustrated in Figure 4. There was almost no difference in the probability of discharge during the first 7 days. Then, there is a divergence in discharge probability between the two arms starting from Day 9, where the hospitalization length of the placebo arm patients was noticeably greater than the active arm. It is important to mention that the hospital discharge criteria were not based solely on the DOS ranking but were highly dependent on other BAT-mandated procedures.
No patient in the active arm received mechanical ventilation during the duration of the treatment, while two patients in the placebo arm received mechanical ventilation for 48 h and 78 h. There were no differences between the two groups regarding oxygen supplementation and overall oxygen saturation of the patients who received oxygen and those who did not receive it. In addition, no significant differences in physical examinations were observed between the two groups. Only the lung status assessments on days 1 and 4 were different, with significant deterioration in the placebo arm (P=0.074 and P=0.10, respectively). A similar trend was observed in the placebo group, when most participants in the active arm were already discharged from the hospitals after 7 days. More details about typical laboratory tests and examination results are provided in the supplementary documents.
Safety outcome
A total of 25 Adverse Effects (AEs) occurred among 11 patients in the active arm (incidence=18%). However, only one was considered related to the study drug-a constipation case, which is a generic and most common AE associated with any calcium administration, including ACC. In contrast, two serious AEs occurred in the placebo arm, including a case of bacteremia sepsis and one respiratory failure resulting in death.
Additional safety aspects, associated with the active substance and consisting of high doses of calcium and carbonate, were assessed by monitoring the blood levels of calcium and bicarbonate. The total serum calcium levels for both arms were very similar from screening-to-discharge, at the lower side of the normal range (8.1 mg/dl-8.8 mg/dl) in each arm.
Bicarbonate levels may be high since the ACC’s carbonate is spontaneously converted to bicarbonate at the body’s pH range by simple chemical equilibria. Serum bicarbonate levels were similar for both arms at the screening and last day of treatment and were distinctively at the high end of the normal range (22 mEq/L-29 mEq/L). At the screening and last day of treatment, the mean bicarbonate levels were 31 (active) versus 29 (placebo) and 27 (active) versus 29 mEq/L (placebo), respectively. This generic observation is further discussed below.
ACC administered sublingually and by inhalation, concomitantly with BAT, has demonstrated clinically meaningful and statistically significant reductions in COVID-19 progression compared to placebo.
The study was a prospective multicenter, randomized, placebo- controlled trial of hospitalized patients with moderate-to-severe COVID-19. Despite the demographic differences in age and gender, the study’s two arms were comparable at baseline in terms of disease severity, symptoms, vitals, comorbidities, and vaccination history. The Breslow-Day tests for homogeneity indicate that the ACC efficacy was similar across the gender and age subgroups for both the primary and the secondary endpoints. Similarities between the groups were also demonstrated in the subpopulations considered as treatment failures due to ICU admission or death during the study.
Improvement and recovery rates were clinically and statistically higher in the active arm, while ICU transfer or death rates were significantly higher in the placebo arm. The duration of hospitalization was similar for the two groups during the first 8 days, partly due to the mandatory hospitalization required by various BATs. However, differences in extended hospitalization durations were found from Day 9, mainly associated with patient deterioration and ICU admission of patients exclusively in the placebo arm.
The premise for applying ACC as a treatment for SARS-CoV-2 is based on growing evidence for its capability to modulate local pH. Progression of coronavirus infection has been associated with acidotic extracellular and intracellular microenvironments that enhance binding, fusion, and replication at the early stages of the disease [20-25]. Additionally, life-threatening complications of COVID-19, including Acute Respiratory Distress Syndrome (ARDS), sepsis symptoms, and organ failures are also associated with acidosis [23,26,27].
Stabilized ACC has already demonstrated anti-acidosis, anti- inflammatory, and immunomodulatory effects in previous preclinical and early clinical studies. Similar therapeutic activities can be anticipated to culminate into an effective antiviral treatment, especially when associated with organ inflammation, due to the interplay between extracellular acidosis and the immune system [28].
The combination of the ACC nanometric primary particles (between 10 nm and 100 nm in diameter) and enhanced solubility of its stabilized amorphous structure by two orders of magnitude (compared to the crystalline phase) makes ACC a unique treatment substance, attributed to its high absorption via mucous membranes [29]. Hence, ACC can efficiently deliver both the calcium and the alkaline carbonate ions by nanoparticle diffusion through mucous membranes to the body fluids, followed by controlled solubility to tissues suffering from calcium deficiency and acidosis [30].
Calcium also plays a role in the body’s response to virus attack. The intensification of COVID-19 severity was recently linked to low serum calcium levels [28]. This observation is actually reinforced by the calcium level data collected in this study. The causality of the phenomenon with the severity is yet uncertain.
The study also provides additional safety information regarding the use of high doses of ACC including inhalations. Although the ACC absorption is expected to be very high [17], the excessive intake of calcium was efficiently excreted from the body. The low range of calcium values at screening agrees with publications reporting the association of severity shift from mild/moderate to severe/ critical with low levels of calcium in the serum and simultaneously increased levels of proinflammatory factors [31]. Since several publications have associated COVID-19 with low serum calcium, the potential of a therapeutic effect for patients with COVID-19 by administering high doses of calcium cannot be excluded.
The overall high levels of bicarbonate and its reduction in the active arm could be due to overall improvements in the pulmonary function. In addition, since the blood bicarbonate indicates the level of CO2 in the blood, it is expected to be high due to respiratory malfunctions resulting in insufficient expiration of CO2. These observations are supported by recent publications reporting increased bicarbonate levels among COVID-19 patients, with no clear relationship to the blood pH [32].
Overall, several significant efficacy outcomes of the ACC treatment were demonstrated from the perspectives of both improvement rates and prevention of disease deterioration. These conclusions are more definite for patients hospitalized with severe conditions at the screening stage, since this was the predominant stage of the study participants.
The study outcome supports the potential of ACC in treating patients with moderate to severe COVID-19 by enhancing recovery and, most importantly, preventing deterioration to critical or fatal conditions. Since the study was performed with hospitalized patients with evidence of clinical pulmonary inflammation, it did not assess patients with mild conditions at all. Therefore, we cannot exclude its potential efficacy in treating mild SARS-CoV-2 patients.
The encouraging study results and additional efficacy evidence observed in numerous successful compassionate treatments for patients with critical lung functioning deterioration who had not met the inclusion criteria. A future larger study is warranted to confirm the promising effects of ACC in treating corona- type bursts as well as other various lung diseases. The current low global capability to recruit patients in focused geographical locations hinders the rapid deployment of such expanded studies. Alternatively, efficacy studies to treat patients with various severe pulmonary inflammatory diseases seem to be the next obvious extension for building confidence in ACC as an anti-inflammatory drug.
This article is dedicated to the memory of the deceased Dr. Albert El-Roeiy, MD, who participated in the study monitoring and analysis. He was intended to be the leading Author, and originally wrote segments of the introduction and discussion sections.
Drs Yigal Blum, Albert El-Roeiy, and Yafit Stark are (were) contractual consultants of Amorphical LTD. Drs. Abu Saleh and Abu Jabal is full employees of the Ziv Medical Center with no contractual or monitory affiliation with the sponsoring company. Frederic Deutsch is an independent biostatistician that was hired to design, implement and analyze the statistical analysis of the study.
Drs Abu Saleh and Abu Jabal oversaw the COVID-19 unit and its medical team at the Ziv Medical Center during the period of the study. Drs Blum, El-Roeiy and Stark were involved in the study planning at the protocol development stage, explaining the hypothesized ACC mode of action to the various medical teams, post-study data analysis and writing this article.
The study was fully sponsored by Amorphical LTD. No other external funding was provided for the execution of the study and its analysis.
Additional data about the study design and execution is provided in a separate Supplementary, including the Study Protocol, Statistical plan and Statistical analysis report.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Saleh NA, Blum Y, El-Roeiy A, Stark Y, Jabal KA, Deutch F (2023) Sublingual and Inhaled Amorphous Calcium Carbonate for Hospitalized Patients with SARS-CoV-2: A Phase 1/2 Clinical Study. J Clin Trials. 13:538.
Received: 04-Sep-2023, Manuscript No. JCTR-23-25395; Editor assigned: 07-Sep-2023, Pre QC No. JCTR-23-25395 (PQ); Reviewed: 21-Sep-2023, QC No. JCTR-23-25395; Revised: 28-Sep-2023, Manuscript No. JCTR-23-25395 (R); Published: 05-Oct-2023 , DOI: 10.35248/2167-0870.23.13.538
Copyright: © 2023 Saleh NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.