Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Case Report - (2019)Volume 7, Issue 2
Submitral aneurysm is seldom reported worldwide. It is thought to be caused by weakness in the cardiac musculature due to abnormal embryogenesis. Mostly these aneurysms were reported from Africa in black African population. Apart from congenital cause, many other causes have been put forth as its etiology. Among these endocarditis is a known infective etiology. Submitral aneurysm secondary to left sided infective endocarditis is rare. The definitive diagnostic modality and treatment of submitral aneurysm remains echocardiography and surgical correction of aneurysm respectively. The submitral aneurysms as well as infective endocarditis both are known independent risk factors for thromboembolic events and subsequent stroke increasing both morbidity and mortality. The management of submitral aneurysm includes surgical correction by repair of the aneurysm but it requires careful consideration of surgical resection of diseased paravalvular tissue or diseased mitral valve and correction of mitral regurgitation by preforming mitral valve replacement. The surgical approach reduces the risk of recurrent endocarditis and thromboembolic events, thus reducing the risk of stroke. Prior to surgery, patient should receive prophylactic antibiotics for one to two weeks to control any active infective endocarditis. For us successful treatment of a patient suffering from submitral aneurysm due to infective endocarditis was challenging. We are reporting a case of submitral aneurysm secondary to infective endocarditis along with review of literature on infective endocarditis and submitral aneurysma.
Infective endocarditis; Submitral aneurysm; Echocardiography; Duke criteria
Submitral Aneurysm (SMA) is rarely reported worldwide. It is thought to be caused by weakness in the cardiac musculature due to abnormal embryogenesis. Apart from congenital cause, many other causes have been put forth as its etiology. We are reporting a case of submitral aneurysm secondary to Infective Endocarditis (IE) along with review of literature on IE & SMA.
Infective Endocarditis (IE) affects 3-15/1,00,000 population annually with rising incidence but has significant impact on general population and health sector, and is 4th most common deadly infectious entity after sepsis, pneumonia and intra-abdominal abscess [1-7].
In the pre-antibiotic era, the population at risk belonged to young and middle age group; and common risk factors were congenital heart disease and rheumatic heart disease [8]. But in recent times, commonly affected population is older age group and other reported risk factors are venous catheter, hemodialysis, prosthetic valve replacement, immunosuppression and Intravenous (IV) drug use. Increasing IV drug use over last five decades has increased incidence of IE related hospitalizations. In such cases, risk of IE could be right-sided, left-sided or bilateral [9]. Previously, oral Streptococci were the most common causative isolates, which are replaced by Staphylococci in recent times [10-13].
Pathophysiology
The pathophysiology of IE can be divided into 4 stages:-
I. Bacteremia
II. Bacterial adhesion
III. Biofilm production
IV. Vegetation maturation
The process is explained using flow chart- Bacteria enter blood via the mouth, gastrointestinal tract, urinary tract or skin through IV catheter/invasive procedure → Bacteremia
→ Bacterial adhesion to damaged endothelium (extracellular matrix protein) of heart by surface adhesins (mainly gram positive bacteria) facilitated by platelet microthrombi and fibrin [14]
→ Colonization of bacteria → Bacteria gets embaded within extracellular polysaccharide matrix i.e. biofilm [15] → Cycle of bacterial proliferation, thrombosis and inflammatory process leads to formation of mature vegetation.
IE is most commonly caused by Staphylococcus aureus accounting for approximately 30% of cases [11,12] followed by coagulase negative Staphylococci (CoNS) accounting for approximately 10% of cases [16,17]. CoNS can cause Native Valve Endocarditis (NVE) and also known to cause Prosthetic Valve Endocarditis (PVE) [18]. Oral Streptococci accounts for in approximately 20% cases, other Streptococci approximately 10% cases and Enterococci in approximately 10% cases. Zoonoses, fungi and HACEK group organisms (Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species) together account for <5% cases. Other reported rare isolates are Coxiella burnetii, Trophyrema whipplei and Bartonella species [19].
A modified Duke’s criteria used to stratify patients into 3 categories: definite, possible and rejected cases. Typical causative organisms of IE (Viridans Streptococci, Streptococci bovis, HACEK group, Staphylococcus aureus, Enterococci) isolated from two separate blood cultures drawn >12 hours apart or all three or a majority of ≥ to four separate blood cultures showing growth is a major criterion of modified Duke’s criteria. According to AHA 2015 guidelines, single positive blood culture for Coxiella burnetii or anti-phase 1 Ig antibody titre ≥ to 1: 800 is added as major criterion along with a positive serology test for Brucella species (slow growing organism) Bartonella species, Legionella species, Tropheryma whipplei, fungi and Mycobacterium species (require special blood culture media) as minor criteria in modified Duke’s criteria [20].
Early diagnosis and prompt treatment improves mortality rate even in the presence of co-morbidities in the elderly patients shown by studies conducted in Italy and in France [21,22]. Since clinical picture is varied in every case, patients presenting with indolent low-grade fever to acute sepsis, heart failure or stroke which does not really help in reaching early or accurate diagnosis. Other less common findings seen are Osler nodes, Janeway lesion, Roth spots and splinter hemorrhage [23]. The modified Duke criteria has lower sensitivity in PVE and Cardiac Device Infection (CDI) [24,25]. The diagnostic approach depending on clinical suspicion and echocardiography together has reduced time to diagnose IE than by using Duke Criteria and the modified Duke criteria [26].
Echocardiography remains a rapid, reproducible, non-invasive and diagnostic test in many cases [27]. Initial recommended test of choice remains Transthoracic Echocardiography (TTE) in patients with either NVE or PVE with sensitivity of 60%-65%; though specificity of TTE is lower for the diagnosis of PVE (ranges from 40% to 70%) when compared to NVE (specificity around 90%). The sensitivity of Trans-esophageal Echocardiography (TEE) ranges between 85%-98%. In a study by Barbieri et al., a good quality TTE was found sufficient for diagnosis in 80% of cases with low level of clinical suspicion [28].
The use TEE is necessary in cases where TTE is positive/non diagnostic, suspected complications (perforation, abscesses and fistula) or presence of intra-cardiac device leads [28,29].
ACC/AHA valvular heart guidelines recommended use of cardiac CT analysis when the anatomy is unclear following echocardiography [24]. The other advantages of cardiac CT over TEE are better evaluation of para ventricular anatomy complications (like mycotic aneurysm/para ventricular abscesses) as well as fewer prosthetic valve artifacts [30-32].
Newer modalities like 18-Fluorodeoxyglucose Positron Emission Tomography (FDG PET) or leukocyte scintigraphy [radio-labeled leukocyte Single-Photon Emission Computed Tomography (SPECT)] have potential to increase detection rate and changes diagnosis from ‘possible’ to ‘definite’ IE [33-35].
Use of CT imaging for detection of para-valvular lesions and use of [18] FDG PET/ SPECT for PVE is now considered as a major criterion in 2015 ESC guidelines [36]. The use of FDG PET/ SPECT has increased the sensitivity of the Duke criteria without affecting specificity [18,37,38].
IE remained untreatable until the beginning of penicillin use [39,40]. In 1955, AHA advocated use of antibiotic prophylaxis before invasive medical and dental procedures with sole intention of IE prevention. However, antibiotic prophylaxis is restricted to population at high risk of IE and its complications, undergoing invasive dental procedures by AHA in 2007 and by ESC in 2009 whereas U.K. National Institute for Health and Care Excellence (NICE) has recommended no use of antibiotic prophylaxis in 2008. An analysis of medical health care data from 2003 to 2015 by Thorhill et al. has found that a significant increase in IE incidence among high-risk population and a borderline significant increase in moderate-risk population after updated recommendations for antibiotic prophylaxis by AHA in 2007 [41]. The effective and safe treatment for uncomplicated IE by oral Streptococci is a combination of aminoglycoside with penicillin/ceftriaxone for 14 days [42]. An uncomplicated right sided IE by Methicillin Sensitive Staphylococcus aureus (MSSA) is effectively treated by a two week course of penicillin mono-therapy [43]. According to ESC & AHA guidelines for treating methicillin resistant Staphylococcus aureus (MRSA)/MSSA causing NVE, aminoglycosides are no more recommended. IE due to aminoglycoside resistant Enterococcus faecalis can be treated with ampicillin and ceftriaxone [20,36].
Daptomycin use was found as effective as conventional therapy (i.e. vancomycin/penicillin with gentamycin) in treating Staphylococcus aureus bacteremia and right sided IE with added advantage of less renal dysfunction (11% of cases) than conventional therapy (26% of cases) [44,45].
Surgery as treatment option is indicated in cases of high risk of embolism or recurrent embolism, uncontrolled infection, progressive valve and tissue damage, and valve dysfunction causing heart failure [46,47]. Stroke is seen in 20%-40% of IE cases complicating the scenario and noted risk factors for embolisation are Staphylococcus aureus infection, mitral valve involvement, mobile vegetation, vegetation size more than 10-15 mm [48-52] .
Mitral valve anatomy
Mitral valve separates Left Ventricle (LV) from Left Atrium (LA). Mitral annulus of the valve has two parts namely anterior and posterior. The anterior annulus is attached to aortic annulus forming aorta-mitral curtain [53].
The posterior annulus has rim of fibrous tissue interrupted by fat enabling its remodeling (enlargement) [54,55]. The posterior annulus has external part (related to LV musculature) & internal part (related to LA); & at junction of both parts it merges with the hinge of Posterior Mitral Leaflet (PML) [53].
SMA externally bulge behind the left Atrio-Ventricular (AV) groove [56] & internally communicates with LV more often than LA [57,58] (Figure 1).
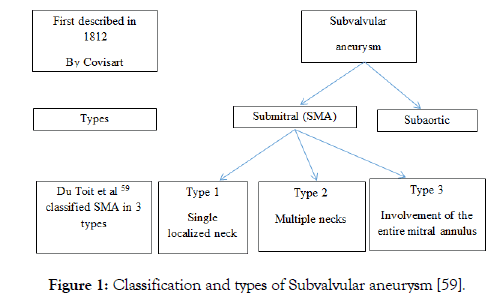
Figure 1: Classification and types of Subvalvular aneurysm [59].
Pathophysiology
SMA is a rare clinical entity and has reported most often in the African black population [60-62]. This suggests that black population is congenitally predisposed to SMA [61]. But such a racial predilection is relative because cases have been reported from other races around the world. The underlying pathology is defective union of the left ventricular musculature with the atrio-mitral valve area creating potential weak region for formation of aneurysm. Such a defective heart could be a result of abnormal embryogenesis in first trimester of pregnancy. The potential weakness can remain confined to small area measuring few millimeters or can extend to the whole of the posterior mitral annulus at the muscular fibrous junction. The study by Nayak VM on normal human hearts autopsy supported sub-mitral curtain as a congenital cause for SMA [63]. This weakness allows hematoma formation which gets organized over a period of time contained within fibrous walled epicardial aneurysm. A false aneurysm thus formed may expand under high pressure in left ventricle [64,65].
On the contrary, some believe congenital SMA is a true aneurysm since aneurysmal wall is constituted of myocardial tissue [66].
Trauma, infection, inflammation and iatrogenic causes are other known causes of acquired SMA. Acquired infective causes of SMA have been reported as tuberculosis [59,67,68], rheumatic endocarditis [59], acute rheumatic fever [69], mitral valve endocarditis [70], infective endocarditis, syphilis [71], trypanosomiasis [56] whereas polyarteritis nodosa, Takayasu arteritis [72] are inflammatory causes. Among the iatrogenic causes mitral valve replacement, and as a sequela to electrophysiology studies have been reported previously [73].
The previously described effects of large aneurysm are-
I. Restricted leaflet movement
II. Mitral Regurgitation (MR) due to displacement of PML & the subvalvular apparatus
III. Circumflex artery compression causing angina pectoris or ventricular arrhythmia
IV. Congestive Heart Failure (CHF) due to MR
V. Peripheral embolic events [58]
a. Cardiomyopathy [74]
b. Compression of left main coronary artery (seen in severe mitral regurgitation) [75]
c. Complete heart block [76]
d. Sudden cardiac death [75]
Usually ECG and chest X-ray are non-specific hence do not help in SMA detection. ECG may show sinus rhythm, ST segment/T wave changes, sinus tachycardia, left ventricular hypertrophy, supraventricular tachycardia, atrial fibrillation, low voltage complexes, signs of ischemia. Chest radiography reveals variable degree of cardiomegaly, signs of pulmonary congestion, aneurysmal calcification. Cardiac catheterization and ventricular angiography were the only option to study aneurysms before the availability of echocardiography [77].
Echocardiography (2D, 3D, colour Doppler, contrast, transthoracic and trans-esophageal) helps to detect and study SMA more readily than ever before [78]. Cardiac CT/MRI provides much more detailed picture of SMA as well detects coronary artery compression, thrombus formation and calcification associated with aneurysm [77].
A 45 year old male presented with history of dyspnea on exertion (NYHA class 3) since one month. Patient was hemodynamically stable on admission. He had no co-morbidities. He was tobacco chewer (for 18-20 years), smoker (4-5 cigarettes/day for 15-16 years) and chronic alcoholic (for 10 years) with very poor oral hygiene. He has six siblings with no history of heart condition or history of sudden death among family members.
The ECG showed sinus tachycardia and suggested Left Ventricular Hypertrophy (LVH) (Figure 2).
Figure 2: ECG on admission showed sinus tachycardia with LVH. (LVHLeft Ventricular Hypertrophy).
The chest X-ray revealed cardiomegaly. He underwent 2D echocardiography with color Doppler study which revealed thickened, prolapsed anterior and posterior mitral leaflets, severe mitral regurgitation (eccentric), multilobed submitral aneurysm, mild left ventricular systolic dysfunction (ejection fraction 55%) and vegetation attached to Anterior Mitral Leaflet (AML) measuring 16×9 mm (Figure 3).
Figure 3: Transthoracic echocardiography showing cyst like structure (i.e. part of aneurysm) extending from left ventricle and second picture showing multilobed nature of submitral aneurysm. (LV-Left ventricle; LALeft Atrium; RA-Right Atrium; A-Aneurysm).
SMA was communicating with Left Ventricle (LV) confirmed by color Doppler. A to and fro blood flow was noticed during color Doppler (Figure 4).
Figure 4: Color Doppler showing blood flow between LV & aneurysm. LV-left ventricle.
Further LV angiogram was performed which revealed filling of multilobulated cystic spaces below mitral valve (Figure 5). This further confirmed the diagnosis of SMA. Patient underwent mitral valve replacement with 27 mm St Jude mechanical valve and surgical repair of SMA. The vegetation grown on AML was sent for culture and sensitivity which grew methicillin resistant CoNS. Histopathology study of resected mitral valve specimen did not suggest any inflammatory etiology. Patient recovered well after surgery without mitral regurgitation at the time of discharge.
Figure 5: LV angiogram showed multilobed submitral aneurysm which is delineated in second picture.
• Submitral aneurysm secondary to infective endocarditis is a rare clinical entity.
• Poor oral hygiene should be considered as risk factor and evaluated by echocardiography to rule out infective endocarditis.
• Submitral aneurysm requires surgical correction of aneurysm and mitral valve replacement to correct mitral regurgitation.
• Presence of infective endocarditis complicates management of submitral aneurysm due to risk of stroke and possibility of spread of infection to aneurysmal cavity further increasing the risk of thromboembolic events.
Citation: Shakapur C, Honnalli M, Mali S (2019) Submitral aneurysm secondary to infective endocarditis: Review of literature and case report. Angiol Open Access 7:229
Received: 05-Apr-2019 Accepted: 17-May-2019 Published: 24-May-2019 , DOI: 10.35248/2329-9495.19.7.229
Copyright: © 2019 Shakapur C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.