Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2020)Volume 10, Issue 4
Background: Achalasia is classified by high-resolution manometry into three subtypes, which are proposed to predict clinical outcome.
Goals: The aim of this prospective study was to evaluate the clinical outcomes of achalasia subtypes after pneumatic dilation, their manometric and radiologic features.
Results: Of the 53 patients, 07 (13%) were classified as subtype I, 44 (83%) as subtype II, and 2 (4%) as subtype III. Clinical response among the subtypes were similar: 7/7 (100%) subtype I, 39/44 (88,64%) subtype II and 2/2 (100%) subtype III. Forty-four patients were submitted to pre- and post-treatmenthigh-resolution manometry. The integrated relaxation pressure and the basal respiratory pressure of the lower esophageal sphincter were significantly lower after the treatment (p<0,001), with a similar decrease between subtypes I and II (p=0,494 and p=0,608, respectively). Logistic regression analysis found that elevated integrated relaxation pressure and basal respiratory pressure of the lower esophageal sphincter were associated with high integrated relaxation pressure after pneumatic dilation (OR 1.13 and 1.04, respectively). Barium column height, at timed barium esophagram , at minute 5 was higher than 5 cm in 18/27 (66.6%) patients with clinical response and in 2/3 (66.6%) patients without clinical response (p=1.00).
Conclusion: No difference in clinical response to pneumatic dilation was observed among the 3 subtypes. Barium column height and the manometric features studied were not related with clinical outcome.
Achalasia; High-Resolution Manometry; Endoscopic Pneumatic Dilation; Timed Barium Esophagram
Achalasia is a motor disorder of the esophagus, which may be a primary disorder (idiopathic achalasia) or secondary to Chagas' disease, with a prevalence of 10 cases per 100.000 population [1,2]. The most common symptoms are dysphagia and regurgitation, although pain and weight loss may also be observed [3,4]. The diagnosis is established based upon the clinical manifestations and complementary tests: upper digestive endoscopy, barium esophagogram, and esophageal manometry, the latter being considered the gold standard [2-6].
Esophageal pressure topography associated with high resolution manometry (HRM) has been recently introduced in medical practice, with advantages over conventional manometry, such as higher accuracy in the diagnosis of esophageal motor disorders and easy procedure execution [7]. According to Bansal and Kahrilas achalasia diagnosed by HRM allows the definition of three manometrically distinct subtypes, which were suggested to have prognostic implications [8]. The three subtypes must have impaired lower esophageal sphincter relaxation on deglutition (integrated relaxation pressure ≥ 15 mmHg) and one of the following body defects: subtype I (classical achalasia), 100%
aperistalse in the esophagus; subtype II (panesophageal pressurization), panesophageal pressurization higher than 30 mmHg in at least 20% of swallows; and subtype III (spastic achalasia), premature waves in the distal esophagus in at least 20% of swallowing [9].
However, it is interesting to consider that most studies evaluating the clinical response of achalasia subtypes to the treatments available did not have homogeneous results [10-18].
The objective of the present study was to analyze the clinical responses of patients with achalasia after endoscopic pneumatic dilation procedure. As such, the HRM findings of the three achalasia subtypes and the timed barium esophagram (TBE) were analyzed.
Patients
From May 2015 to March 2018, were enrolled consecutive non treated patients diagnosed with achalasia by HRM from the Ambulatory of Esophageal Diseases at Policlinica Piquet Carneiro, State University of Rio de Janeiro, Rio de Janeiro, Brazil.
The study was approved by the Ethics Committee of the State University of Rio de Janeiro. The research protocol conforms to Declaration of Helsinki. Patients were over 18 years old and signed the informed consent form to participate in the study.
Exclusion criteria were previous treatment for achalasia or previous gastroesophageal surgery, pseudoachalasia, sigmoid megaesophagus, nasal surgery in the last 6 months. Chagas' disease patients (defined by positive serology), pregnancy, coagulopathies, psychiatric disease, and x(ESS) lower than 3, were also excluded.
The enrolled patients were interviewed by the same physician and were evaluated for gender, age, and weight. In addition, a clinical questionnaire with external validation, the ESS was answered, which assesses the therapeutic response in achalasia [19]. The ESS attributes a score for dysphagia (0, absent; 1, occasionally; 2, daily; and 3, each meal), regurgitation (0, absent; 1, occasional; 2, daily; and 3, each meal), chest pain (0, absent; 1, occasional; 2, daily; and 3, each meal), and weight loss (0, no weight loss; 1,5 kg; 2, 5-10 kg; and 3,10 kg).
High resolution manometry
A 36-channel, solid-state sensors at 1cm intervals, catheter system was used to perform the HRM (Given Imaging, Duluth, USA). Data were acquired using a dedicated software ManoScanᵀᴹ ESO Acquisition. HRM studies were performed after at least a 6h fast. The catheter was placed transnasally and positioned to record from the hypopharynx to the stomach. The manometric protocol included a 5 min period to assess basal low LES pressure and 10 swallows of 5 mL of water at 30s intervals in the supine position. ManoViewᵀᴹ ESO Analysis.0020 software was used to analyze the data. Manometric variables included lower esophageal sphincter basal pressure, integrated relaxation pressure (IRP), distal latency (DL), and distalcontractile integral (DCI). These variables, per the Chicago classification v3.0, were used to determine achalasia in each patient [20]. A gastroenterologist trained in HRM reviewed all of the tracings.
Achalasia was diagnosed If there was no peristalsis, panesophageal pressurization, or premature contraction, and the IRP was ≥ 15 mmHg, and then it was classified into 3 subtypes (type I, II, and III) based on the Chicago Classification v3.0 [9].
Upper gastrointestinal endoscopy
Upper gastrointestinal endoscopy (UGE) (Fujinon, EG 450HN, Tokyo, Japan) was performed to rule out pseudoachalasia and other pathologic findings that contraindicated pneumatic dilation (PD). The procedure was performed with monitoring (pulse oximetry and cardioscope); moderate sedation (conscious sedation) with midazolam, meperidine and propofol in fractional doses, titrated according to the individual need of each patient. Xylocaine® spray, 10%, was used for topical anesthesia of the oropharynx. UGE was performed by an endoscopist with the presence of the primary investigator, and a nursing technician.
Pneumatic dilation
PD was performed provided obstructive and or mass lesions were discarded by UGE. A 30 mm Rigiflex balloon (Boston Scientific, USA) was positioned through a metal guidewire (Savaryguidewire), with direct endoscopic view, at the esophagogastric junction (EGJ) and dilated at a pressure of 8 PSI for 1 minute.
Timed barium esophagogram
One month after the PD, TBE was performed in patients as follows: after deglutition of 100 mL of barium diluted at 50% ingested in 15 to 30 seconds, contrasted radiographs in the posterior left oblique erect position were performed at minutes 1, 2, and 5 [21]. The height of the barium column was measured at the first and fifth minutes, as well as the maximum diameter of the esophagus. All the exams were performed and evaluated by the same radiologist, from the Radiology Department of the State University of Rio de Janeiro. Test was considered abnormal if the height of barium column was>5 cm at 5 min [22].
Study protocol
Patients with complaints such as dysphagia, chest pain, and regurgitation, were invited to participate in the study. A consent form was signed before each procedure. The enrolled patients underwent the following procedures: clinical evaluation, UGE, PD, two HRM procedures (pre-treatment and another one month after PD) and TBE.
At the first interview, patients completed a clinical questionnaire that included the ESS. They were submitted to HRM, and serology for Chagas’ disease was requested. Once there was a positive diagnosis of achalasia at the HRM and Chagas’ disease was excluded, patients were then included in the study.
Asserted by UGE that there were no contraindications, PD was performed, using a 30 mm pneumatic balloon.
Thirty days after PD, there was a follow-up interview with clinical evaluation, including ESS and it was performed a new HRM and TBE. If ESS was ≤ 3, which indicates therapeutic response, a new visit was scheduled 6 months after PD, with a new clinical evaluation by the ESS. If the patient presented ESS>3 (indicative of therapeutic failure) at the first follow-up visit, a new PD was performed with a 35 mm balloon. One month after this second PD, the patient was reassessed and if the ESS remained>3, athird and last 35 mm balloon dilation was performed. If ESS remained>3 after the latter dilation, patient was considered to have a therapeutic failure and was referred to surgery department in order to evaluate surgical myotomy.
Statistical analysis
Quantitative variables are shown as mean ± standard error (SE) or median (range) and qualitative variables are shown as absolute number and frequency (%). Comparisons between the independent groups were performed using the chi-square test (chi2) for categorical variables and the Mann-Whitney test for continuous variables. McNemar test and paired Wilcoxon test were used for categorical and numerical variables, respectively, in the analysis of paired samples (repeated measures). Logistic regression was performed to identify risk factors associated with the outcome as well as a description of their odds ratios and 95% confidence interval. pvalues<0.05 in bilateral tests were considered statistically significant. Statistical analyzes were performed using Stata software for Windows (version 15, StataCorpLP, USA).
Patient characteristics
Forty-six patients (90%) achieved clinical response (ESS ≤ 3) after the first PD with the 30-mm balloon. When all dilations were included, with 30- and 35-mm balloons, clinical response achieved 98%. One patient was considered a therapeutic failure (subtype II) and was referred to surgery in order to evaluate for laparoscopic Heller myotomy (LHM).
Of the 53 patients included, 7 (13%) had achalasia subtype I, 44 (83%) had subtype II, and 2 (4%) had subtype III (Figure 1). Considering the small number of patients with subtype III, data analysis of this group was only descriptive. Statistical analyses were based on the comparison between subtypes I and II: 51 patients included for clinical analysis and 44 for manometric analysis.
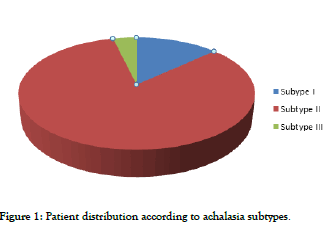
Figure 1: Patient distribution according to achalasia subtypes.
Of the 51 patients, 35 were female (68%) and 16 were male (32%). The median age of patients was 52 years old (IQR 43 to 64). The median pre-PD weight was 62 Kg (IQR 56 to 75). There was no statistically significant difference in age and gender distribution between the two subtypes.
Clinical response
The overall clinical response after the first PD using the 30-mm balloon was 90%. Clinical response was defined by an ESS ≤ 3 after the PD. When all dilations were included, with 30- and 35- mm balloons, clinical response achieved 98%. Only one patient with achalasia subtype II failured therapy, and was sent to surgery department for LHM. All statistical analyses were performed after the first PD, to standardize the sample.
After the dilation treatment, there was a significant decrease in ESS: median pretreatment 7 (IQR 6-9) vs. post-treatment 0 (IQR 0-1; p<0.001). There was no difference in clinical response between the subtypes: median decrease in subtype I ESS, 7 (IQR 5-11) and subtype II ESS, 6 (IQR 5-8; p=0.39). Both groups had a satisfactory clinical response, with ESS ≤ 3 in 7/7 (100%) subtype I patients vs. 39/44 (88.64%) subtype II patients (p=0.35; Table 1).
| Achalasia subtype | |||
|---|---|---|---|
| Clinical response (n) | Subtype I | Subtype II | Total |
| 7 | 39 | 48 | |
| 100.00% | 88.64% | 90.20% | |
| p value=0.348 | |||
| Weight gain (n) | 5 | 34 | 39 |
| 83.33% | 79.07% | 79.59% | |
| p value=0.808 | |||
Table 1 : Patient distribution according to achalasia subtypes.
Weight gain was also significant with median pretreatment 62 (IQR 56 – 75.4) vs. median post-treatment 67 (IQR 60 – 76; p<0,001), with no difference between the two subtypes
(p=0,808) (Table 1). No independent factors, such as gender, age, achalasia subtype, pretreatment IRP and pretreatment basal LES pressure, were associated with better clinical outcome or weight gain.
There were two perforations during PD procedures among 66 dilations (3%), one with the 30-mm balloon and another with the 35mm balloon. Both patients were treated accordingly and fully recovered.
Manometry analysis
Of the 51 patients distributed between subgroups I and II, 44 underwent pre- and postdilationmanometry. The analysis of the manometric data showed a significant decrease in IRP and basal EEI pressure after PD in both groups (p<0.001), with no statistically significant difference between them (IRP: p=0.494; basal pressure: p=0.608) (Tables 2 and 3).
| Median | IQR | |
|---|---|---|
| IRP predilation | 33.15 | [28.1-38.6] |
| IRP postdilation | 14.4 | [10.4-25.5] |
| Variation | [-14.3] | [-9.0,-20.6] |
| p<0.001 | ||
| Predilation basal LES pressure | 46.4 | [37.2-57.0] |
| Postdilation Basal LES pressure | 30.2 | [21.0-39.2] |
| Variation | [-14.1] | [+7.1, -28.7] |
| p<0.001 |
IRP: Integrated Relaxation Pressure; LES: Lower Esophageal Sphincter; IQR: Interquartile Range
Table 2 : decrease in IRP and basal LES pressure.
| IRP | Median | IQR |
|---|---|---|
| Subtype I (n=6) | ||
| Variation postdilation | [-12.4] | [-4.5,-18.1] |
| Subtype II (n=38) | ||
| Variation postdilation | [-15.1] | [-9.8,-20.6] |
| p value= 0.494 | ||
| Basal LES pressure | Median | IQR |
| Subtype I (n=6) | ||
| Variation postdilation | [-20.1] | [-5.40,-36.8] |
| Subtype II (n=38) | ||
| Variation postdilation | [-14.1] | [-7.7,-27.1] |
| p value=0.608 |
IRP: Integrated Relaxation Pressure; PD: Pneumatic Dilation; LES: Lower Esophageal Sphincter
Table 3 : variation in IRP and LES basal pressure values before and after PD in both subgroups, I and II.
Postdilation IRP was lower than 15 mmHg in in 23/44 (52%) patients: 4/6 (66.7%) subtype I patients and 19/38 (50%) subtype II patients (p=0.448). High pre-dilation IRP and high basal LES pressure were associated with elevated postdilation IRP (>15 mmHg): IRP–OR=1.13 (95%, CI 1.03-1.24), p=0.009 and basal LES pressure OR=1.04 (95%, CI 1.01-1.08), p=0.049.
There was no association between clinical and manometric responses, i.e., patients with postdilation IRP ≤ 15 mHg did not have higher clinical response with ESS ≤ 3 (p=0.563).
Of the 53 patients included, 7 refused to perform the HRM post-PD. Of the 44 patients with HRM before and after, 25 (56.8%) had a change in achalasia subtype after dilation: 23 (52.2%) from subtype II to I; one (2.2%) from II to III; and one (2.2%) I to II.
Radiologic analysis
Thirty patients underwent TBE after PD. The values of esophageal diameter and height of barium column at minutes 1 and 5 according to the presence or absence of clinical response are shown in Table 4. There was no statistical difference between responders vs. non-responders regarding the radiological parameters recorded (Table 4).
| Non-Clinical response | Clinical response | ||||
|---|---|---|---|---|---|
| n=3 | n=27 | ||||
| Mediana | IQR | Mediana | IQR | p valor | |
| Diameter 1 min (cm) | 4.6 | [4.0-5.0] | 3.3 | [2.0-4.9] | 0.226 |
| Diameter 5 min (cm) | 4 | [2.0-4.7] | 2.3 | [1.2-4.3] | 0.35 |
| Barium column height 1 min (cm) | 13.5 | [5.7-15.1] | 9.2 | [4.9-11.6] | 0.254 |
| Barium column height 5min (cm) | 12.6 | [2.0-21.7] | 7.5 | [2.5-10.3] | 0.351 |
| Diameter variation 1min,5min (cm) | [-0.60] | [-0.30, -2.00] | [-0.50 | [-0.20, -1.00] | 0.368 |
| Barium column height variation 1min,5min (cm) | [-0.90] | [+6.6, -3.7] | [-0.90] | [-0.10, -3.8] | 0.678 |
Table 4 : Radiological variables analyzed. Comparison of radiological findings between patients who responded clinically (Eckardt score = 3) and those who did not respond (Eckardt score>3).
When barium column was evaluated at minute 5 using a 5 cm height as cutoff, there still was no difference between patients who responded clinically vs. those who did not respond (p=1,000), as well as between those who had a postdilation IRP higher than 15 mmHg vs. those with postdilation IRP ≤ 15 mmHg (p=0.705) (Table 5).
| Barium column height at minute 5 | |||
|---|---|---|---|
| >5cm | = 5cm | Total | |
| No clinical response: Â ESS>3 | 2 (10%) | 1 (10%) | 3 |
| n (%) | |||
| Clinical response: ESS= 3 | 18 (90%) | 9 (90%) | 27 |
| n (%) | |||
| P=1,000 | |||
| IRP postdilation = 15 mmHg | 9 (47,4%) | 4 (40%) | 13 |
| n (%) | |||
| IRP postdilation<15 mmHg | 10 (52,6%) | 6 (60%) | 16 |
| n (%) | |||
| P=0,705 | |||
IRP: Integrated Relaxation Pressure; ESS: Eckardt Symptom Score
Table 5 : barium column height higher than and lower than 5 cm at minute 5: distribution between patients with clinical response vs. nonclinical response and between patients with IRP = 15 mmHg vs.< 15 mmHg postdilation
Subtype III analysis
Of the 53 patients, two had subtype III achalasia. Both of them responded to PD, with postdilation ESS=0. Their IRP and basal LES pressure dropped, but the IRP did not fall under 15 mmHg in neither. Neither gained weight nor performed TBE.
Achalasia is a chronic condition of the esophagus that can present with dysphagia, regurgitation, chest pain and weight loss or secondary respiratory complications [23]. Achalasia results from impaired relaxation of the lower esophageal sphincter and lack of peristalsis in the tubular esophagus without obstructive pathology. Currently, upper endoscopy, TBE, and HRM are used to establish a diagnosis of achalasia and follow treatment outcomes [24]. While these tests are also used to monitor treatment response, a self-reported measure of symptom severity is often the primary outcome in clinical studies and is often the principal factor that drives clinical management decisions [25]. What is unclear is how well these physiological measurements of esophageal function correlate with patient-perceived healthrelated quality of life [26].
HRM is a commonly used tool for assessing patients with achalasia. Such topographical studies allow detailed analysis of esophageal peristalsis and circular smooth muscle contraction and relaxation. A failure of relaxation of the LES is a clinically important issue in the setting of achalasia. This disease is classified in the Chicago classification of esophageal motility disorders v3.0 at the top of the hierarchical assessment of esophageal motility by HRM due to their high association with esophageal symptoms [27]. Whilst HRM analysis according to the Chicago classification has great utility in assessing esophageal motor disorders, it does not always give clear indication as to the degree of any EGJ obstruction and it is a well-documented finding that HRM do not always correlate well to symptoms in patients with achalasia [28]. This event may be due to the inability of HRM to investigate beyond circular muscle contraction, but a further factor may be the unphysiological nature of the tests [29]. The standard ten 5 mL water swallows during HRM are valuable, and have allowed the application of standardized diagnostic criteria in their interpretation [9]. However, there are limitations in confining studies to single swallow parameters. In particular, they are not representative of swallowing in everyday conditions. In contrast, application of solid meal swallows to an HRM protocol may allow for “unmasking” of motility disorders not seen on single swallows, but can be cumbersome to perform and interpret [29]. It is also already known that achalasia can lead to a significant impairment in quality of life and in this way our aim was to assess whether objective physiological measurements of HRM and TBE can predict clinical response to PD treatment in patients with idiopathic achalasia [30]. Overall, subtypes of achalasia determined by HRM did not predict clinical response of PD as symptom outcome was evaluated by ESS.
The main aim of treatment in achalasia is to improve symptoms. Traditionally, PD, LHM and, more recently, POEM are current therapies [31]. ESS is a simple and more commonly used
measure to grade symptom severity for achalasia patients in both clinical and research settings and this score has been used as a predictor of outcome but also as a self-report assessment tool to evaluate clinical response after treatment [32,33]. The ESS consists of one uniform scale of which over 50% of the variance is explained by the dysphagia item. Both weight loss and chest pain account for a significantly smaller amount of the variance (around 10% each) in the ESS [34]. On the other hand, weight loss is the only objectively measured variable on the ESS, so less correlation with subjective responses is somewhat predictable. Around 50% of the items on the ESS are not related to standard physiological assessment of achalasia severity [34]. In our study, PD Treatment was efficient, in particular, with a significant decrease in dysphagia score and increases in weight gain but without difference in ESS measurement between subtypes I and II.
Pandolfino et al. described three manometrically subtypes of achalasia: subtype I (aperistalsis, classic achalasia), subtype II (panesophageal pressurization) and subtype III (premature contraction, spastic achalasia) [35]. The authors suggested that these subtypes of achalasia were distinct in terms of their responsiveness to medical or surgical therapies and could be a predictor of clinical outcome. The results indicated that achalasia subtype II was much more likely to respond to therapy compared with subtype I (OR, 11.2 (95th percentile confidence interval [CI], 2.4–35.6); P=0.002). In contrast, achalasia subtype III was much less likely to respond to therapy than subtype I (OR, 0.24 (95th percentile CI, 0.06–0.92); P=0.044) [13]. Since then, many other studies have been reported [11-18]. Variable results related to clinical outcomes after treatment of the three manometric subtypes of achalasia, which turns this issue controversial [14-16]. These findings have been reproducible by other studies but with different methodologies and, on the other hand, studies have shown no difference in symptomatic outcomes based on achalasia subtypes [10-18]. The IRP is the parameter that should most represent the resistance to esophageal emptying offered by the EGJ, our results indeed demonstrated a significant decrease in IRP and basal LES pressure values after PD in both groups, subtypes I and II with no statistical difference between them. However, there was no correlation between this HRM variable and clinical response as measured by ESS. Moreover, the only two patients diagnosed achalasia subtype III had not normalized their IRP after PD, but both of them achieved good clinical response, with post-dilation ESS of zero. Unfortunately, the too small number of patients precluded statistical analysis of these data.
Another issue is the interchangeability among achalasia subtypes. Pandolfino et al. hypothesized that type I patients tended to present with more severe esophageal dilatation with minimal post-deglutitive shortening, and, conceptually, these patients may represent disease progression from type II with esophageal body decompensation after prolonged outlet obstruction[13]. Thus, subtypes I and II achalasia represent different stages of the same disease, with subtype II being an early stage of the disease. Lee et al. also reported on two patients who showed a decrease in esophageal body pressure after a follow-up of approximately 7 years and suggested that this could represent a progress from subtype II to I [11]. In our study, 23 of 25 patients progressed from subtype II to I after PD, all of them with reduction in the EES. Different from Pandolfino´s hypothesis, we suggest that this change in achalasia subtype is due to a post-dilation reduction in the functional obstruction at the EGJ that probably concurred to reduce stress pressure upon esophageal body wall and, consequently, reduced signal capture by pressure sensors in the HRM catheter. In addition, one patient evolved from subtype I to II and another one from subtype II to III after PD treatment. Controversy remains with respect to whether these achalasia subtypes correctly represent distinct motor disorders or are simply different points in the progression from a healthy esophagus to end stage achalasia [16]. Further large number of follow-up data is needed
There is no consensus on what is considered failed therapeutic response after achalasia treatment. There is no well-defined marker, and the concept of response/failure is linked to subjective interpretations of symptoms by patients and physicians [24]. As aim of all treatments of achalasia rely on to relieve functional obstruction of LES, evaluation of esophageal emptying post-treatment is a desired need. One of the most useful and frequently implemented tests is the TBE. The improvement in esophageal emptying after successful therapy can be more accurately evaluated by performing TBE which allows an objective assessment of flow across the EGJ, and has been used extensively in achalasia. The ideal goal of treatment in achalasia is to achieve complete esophageal emptying. Such optimal end point, however, may not be achieved due to aperistaltic dilated esophagus [21]. Esophageal stasis and a wide esophageal diameter, measured by TBE, have been shown as risk factors of treatment failure in some studies [36,37]. Rohof et al., in a study with 41 patients with long-standing achalasia, determined that a barium column height higher than 5 cm at minute 5 of the TBE was considered as incomplete emptying of the esophagus and predicted recurrent symptoms, a marker of therapeutic failure [22].
Vaezi et al. used TBE for assessment of esophageal emptying following PD in patients with achalasia. In 72% of patients, symptoms as well as barium height were improved. Lack of symptomatic improvement was highly associated with poor esophageal emptying (<50% improvement in barium height). However, in 30% of patients with near complete symptom resolution, esophageal emptying was poor; and most of them had recurrent symptoms within one year. The authors concluded that TBE proved to be a good objective tool for predicting long term response following PD [36].
We evaluated the height of barium column at minute 5 of patients who achieved clinical response and those who did not, as well as those who had a post-PD IRP higher than 15 mmHg and those who had post-PD IRP lower than 15 mmHg. There was no difference in clinical or manometric response between patients who presented height of barium column higher than 5 cm or those with barium column lower than 5 cm at the minute 5. Therefore, although in our study, only 30 patients performed TBE post-PD treatment, we could not established a correlation between height of barium column post-PD treatment and clinical response. Krieger-Grübel et al. studied 32 patients after treatment of achalasia and also failed to correlation between
TBE results and clinical response [38]. There were no differences between ESS patients with<3vs.>3 points in the percentage of swallows with complete segmental and total bolus clearance and of patients with barium retention at 0.5, 1, 3, and 5 minutes. Moreover, none of the standardized tests as liquid/ viscous swallows measured by the barium retention correlated with symptom severity assessed by the ESS.
Whilst TBE is likely to be helpful in the untreated achalasia patient where sphincter opening is poor, it may be less helpful in the treated patient. Whilst retained barium post treatment appears to predict long term treatment failure, it does not necessarily correlate with symptoms [36]. Furthermore, a slow challenge of liquid barium may flow through a treated sphincter, even if the degree of opening is insufficient for symptom relief in every day conditions [29].
In spite of this issue is beyond the scope of our study, recent studies have shown that a 200 mL rapid drink challenge (RDC) during HRM can help in the diagnosis of esophageal motility disorders [29,39]. A TBE is a high volume, slow speed challenge to the EGJ, and single 5 mL water swallows in HRM assess a high speed but only very low volume challenge to the esophagus and EGJ [39]. The RDC allows a high volume, high speed “ stress test ” to LES opening, that is more representative of symptomatic swallowing than found during the TBE and may be able to provide more clinical relevance and may better predict symptomatology in patients with achalasia (treated or untreated) than other HRM or TBE metrics. Therefore, in a treated achalasia patient, the absence of a column does not necessarily exclude moderate obstruction. In contrast, the presence of an increased IRP during RDC (even in the absence of a column) might imply persistent obstruction [29].
This study has some limitations. It has a limitation stemming from its small sample size, with only two patients with achalasia subtype III. It did not include measures of EGJ distensibility and RDC parameters that could have helped better understand the relationship between EGJ distensibility and clinical response to predict outcome to treatment [29,40]. In the symptom evaluation, the ESS was used. This is well validated in the untreated situation, but was not devised to evaluate the treated situation. However, there is no specific tool to evaluate symptomatic treatment response in achalasia.
In conclusion, PD is an effective treatment for achalasia patients, decreasing IRP, basal LES pressure and normalizing ESS in a majority of achalasia patients both subtypes I and II. In the treated achalasia patient, it is thus far uncertain whether the mean IRP or subtype of achalasia during HRM or height of barium column on TBI can correlate with clinical outcome response or need for retreatment achalasia patients post-PD treatment. Further studies are required to evaluate theses outcome measurements in patients with achalasia according to treatment modalities.
Citation: Fittipaldi SA, Domingues GRDS, Carvalho ATP, Torezani RS, Filho JPPDM, Barbosa HPP etal.(2020) Subtypes of Achalasia Do Not Predict the Clinical Response of Pneumatic 10:316. doi: 10.35248/2165-8048.20.10.316
Received: 25-May-2020 Accepted: 09-Jun-2020 Published: 16-Jun-2020 , DOI: 10.35248/2165-8048.20.10.316
Copyright: © Fittipaldi SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited