Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Case Report - (2020)Volume 11, Issue 3
Importance: Epidermolysis bullosa pruriginosa (EBP) is a rare adult-onset heredo-familial skin disorder. Bullous skin
lesions are triggered by intense pruritus, which is the hallmark of the disease. Eosinophilic infiltrates and elevated IgE
levels in serum and lesions have been reported, but their pathological role is yet to be determined. Although
treatment with anti-IgE therapy (Omalizumab) has been used successfully in autoimmune bullous diseases but not in
EBP to our knowledge.
Observation: We report a case of a 34-year-old female with adult-onset pruritic and blistering disease of the skin. Sub
epidermal blisters with viable roofs and numerous epidermal neutrophils and eosinophilic infiltrate were detected
histopathologically; however, the absence of IgA, IgG, IgM, C1q, and C3 deposits made the diagnosis of
Epidermolysis bullosa acquisita and Bullous pemphigoid uncertain. Whilst testing for intra-lesional IgE
autoantibodies was not performed, total and specific serum IgE concentrations increased during her illness in the
absence of an allergic or parasitic disease. Because no improvement in her symptoms was observed with conventional
treatments, whole exome sequencing was performed which showed a non-conservative Glycine substitution in the
G2481D residue in the COL7A1 gene suggestive of EBP. Off-label use of anti-IgE drug (Omalizumab) was attempted
due to severity of her symptoms and elevated levels of IgE. On initiating the treatment, the patient showed a
significant improvement in her skin condition; however, a trial to taper off Omalizumab two years later was
unsuccessful.
Conclusion: This case suggests a possible role of IgE autoantibodies in EBP that requires further research,
consolidated by the fact that our patient showed improvement with anti-IgE therapy. Furthermore anti-IgE therapy
offers a possible new targeted treatment for EBP in the absence of curative treatments.
Autoimmune bullous disease; Dystrophic epidermolysis bullosa; Epidermolysis bullosa pruriginosa; IgE autoimmunity; Omalizumab
Dystrophic epidermolysis bullosa (DEB) is caused by point genetic mutations in the COL7A1 gene, which encodes the anchoring fibrils of the basement membrane to the underlying dermis; this mutation causes skin fragility and renders it more susceptible to friction [1].
Epidermolysis bullosa pruriginosa (EBP) (OMIM #604129) is one of DEB rare clinical phenotypes. Intractable pruritus and prurigo-like lichenified nodules are cardinal features of the adult disease form. Pruritus, usually precedes or co-exists with the severe clinical phenotype [2]. Identical COL7A1 gene mutations could cause DEB or EBP clinical phenotype [3], which suggests an additional unknown immunological or environmental mechanism. Rarity of the disease, a variable age of onset and clinico-histological resemblance to other skin conditions makes the diagnosis challenging [4]. Treatment options are limited to a few medications with a high side effect burden making the treatment of pruritus even more difficult [5].
A 34-year-old woman presented to the dermatology clinic with a one-year history of pruritic bullous lesions after minor trauma to the skin. She had a past history of a resected thyroid papillary carcinoma and no childhood atopic disease. Her aunt and a maternal cousin also had bullous skin lesions. On examination, Lichenoid papules were noted with a few vesicles, along with blisters on extensor surfaces of upper and lower limbs, sparing nail, scalp, and mucous membranes (Figure 1). A punch skin biopsy revealed subepidermal blister with a viable roof, epidermal and upper dermal oedema, and perivascular lymphocytic, neutrophilic, and eosinophilic infiltrates. Immunofluorescent assays for IgA, IgG, IgM, C1q, and C3 as well as serum epidermal IgG, celiac disease antibodies, and autoimmune workups were negative. Systemic glucocorticoids at a concentration of 0.5 mg/kg, along with cyclosporine and dapsone, failed to control her symptoms. Repeated skin biopsies were inconclusive. Serum total IgE increased to 2587 ku/l (357 ku/l at baseline). Specific IgE titers for the dermatophagoids pteronyssinus and farinae were 2.23 Ku/L and 5.08Ku/L, respectively. Whole exome sequencing (XomeDxPlus- NY, USA) showed a G2481D variant homozygous, non-conservative amino acid substitution mutation in the COL7A1 gene. This variant has not been identified in the literature as a pathogenic or benign polymorphism; however, in silico analysis predicted a protein-damaging role. As her parent’s DNA samples were not analyzed, a novel mutation could not be confirmed.
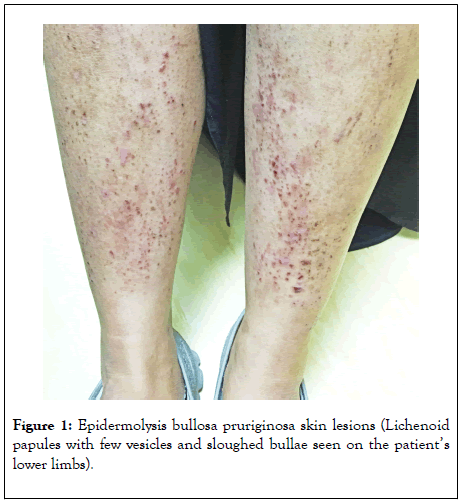
Figure 1: Epidermolysis bullosa pruriginosa skin lesions (Lichenoid papules with few vesicles and sloughed bullae seen on the patient’s lower limbs).
Omalizumab (anti-IgE) was administered in 300 mg subcutaneous injections every four weeks, resulting in a dramatic improvement in pruritus and bullae. Resolution of symptoms was maintained for four years and attempts to stop Omalizumab caused disease relapse.
Self-antigens instigating IgE-mediated autoimmune diseases were recently described [5]. Bullous Pemphigoid (BP) was among the first of the autoimmune skin diseases demonstrating IgE’s selfreactive role. The presence of auto-reactive IgE-antibodies against transmembrane protein BP180 and intracellular protein BP230 and IgE-coated mast cells in perilesional skin with BP180 peptides on these mast cells [6] support the involvement of IgE autoantibodies in the pathogenesis of BP.
Increased concentrations of serum and intra-lesion IgE were repeatedly observed in EBP cases [2,7,8], however a link to disease pathogenicity has not been established yet. Severe itching, which is central to a diagnosis of EBP, in the context of increased IgE suggests a causative role of IgE, particularly when skin lesions are provoked or increased by pruritus. Histamine which is a well-known pruritogen is released primarily by mast cells, when an allergen binds to the IgE receptor on their surface [9]. Auto-reactive IgE have recently been identified in Epidermolysis bullosa acquisita, which was previously viewed as a disorder reminiscent of Dystrophic epidermolysis bullosa (DEB) [10]. Similarly in EBP, autoimmune IgE could be the potential culprit triggering the inflammatory cascade in those who are genetically susceptible.
Omalizumab blocks free serum IgE from binding to the highaffinity FcɛRI on mast cells. It has been licensed for treatment of chronic spontaneous urticaria (CSU) and postulated mechanisms include reducing the activity of IgG autoantibodies against FcɛRI, reducing the activity of auto-reactive IgE against unknown antigens and reducing intrinsically abnormal IgE [11]. Omalizumab has been used to treat BP successfully in the past [12]. Despite this, no data is available on the effects of Omalizumab in the treatment of EBP.
The current case is the first in our knowledge, to be genetically confirmed as EBP and successfully treated with Omalizumab. The diagnosis of EBP was initially challenging due to the lateonset presentation and lack of other supporting features like nail dystrophy. The improvement in bullous skin lesions and disabling pruritus after Omalizumab treatment further supports the etio-pathological role of IgE. In patients with EBP, increased serum IgE in the absence of obvious allergic or parasitic disease may support the use of anti-IgE therapy when conventional therapy fails.
Authors would like to acknowledge Dr. Mansoor Ali Hameed for proofreading the manuscript. No funding organization or sponsoring body has been involved in the design and conduct of the study.
Author and coauthors have no financial disclosures to declare.
Citation: Taha SA, Al-Nesf MA, Al-Obaidli AM (2020) Successful Treatment of Epidermolysis Bullosa Pruriginosa with Anti-IgE Therapy (Omalizumab): A Case Report and Four Years Follow Up. J Clin Exp Dermatol Res. 11:520. DOI: 10.35248/2155-9554.20.11.520
Received: 01-May-2020 Accepted: 14-May-2020 Published: 21-May-2020 , DOI: 10.35248/2155-9554.20.11.520
Copyright: © 2020 Taha SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.