Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Commentary - (2025)Volume 15, Issue 5
Surface tension is a fundamental property of liquids that plays an important role in various natural and industrial processes. It can be observed in everyday life, from water droplets forming perfect spheres to insects walking on water. Surface tension refers to the cohesive force at the surface of a liquid that causes it to behave as if it has a thin elastic skin. It results from the attractive forces between the molecules within the liquid, particularly those at the surface. In a liquid, molecules experience cohesive forces with their neighbors. However, molecules at the surface have fewer neighbors, leading to an imbalance in these forces. This imbalance results in surface tension, causing the liquid to minimize its surface area.
Molecular explanation of surface tension
At the molecular level, surface tension arises due to the difference in the forces experienced by molecules at the surface and those inside the liquid. In the bulk of the liquid, molecules are surrounded by other molecules and the attractive forces are balanced in all directions. However, at the surface, molecules have fewer neighboring molecules on the air side, leading to stronger forces between the molecules on the liquid side. This results in the liquid surface behaving as though it were under tension. The molecules at the surface are pulled inward, minimizing the total surface area. This is why droplets of liquid tend to form spheres, as a sphere has the smallest surface area for a given volume.
Mathematical description of surface tension
Surface tension (denoted by the symbol γ) is defined as the force per unit length acting along the surface of a liquid. It is measured in units of force per length (N/m in the SI system). The formula to calculate surface tension is:
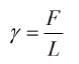
Where,
• γ is the surface tension,
• F is the force acting along the surface, and
• L is the length over which the force acts.
For a liquid droplet, the pressure difference across the surface is given by the Young-Laplace equation:
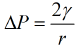
Where,
• ΔP is the pressure difference between the inside and outside of the droplet,
• γ is the surface tension, and
• r is the radius of the droplet.
This equation explains why smaller droplets have a higher internal pressure due to the curvature of their surface.
Factors affecting surface tension
Several factors influence surface tension, including temperature, impurities and the nature of the liquid-
Temperature: As the temperature of a liquid increases, its surface tension decreases. This happens because higher temperatures increase the kinetic energy of the molecules, weakening the cohesive forces between them. Eventually, at the boiling point of the liquid, the surface tension becomes very low.
Impurities: The presence of impurities in a liquid can either increase or decrease its surface tension. For example, adding soap or detergent to water decreases surface tension by disrupting the cohesive forces between water molecules. This property of soaps is what makes them effective at cleaning, as they allow water to spread more easily over surfaces.
Nature of the liquid: Different liquids have different surface tension values due to the nature of the intermolecular forces present. For example, water, which has strong hydrogen bonding between its molecules, has a higher surface tension compared to liquids like alcohol or oil, which have weaker intermolecular forces.
Examples of surface tension in nature
Water droplets: One of the most common examples of surface tension is the formation of water droplets. When water is released from a tap or a syringe, it tends to form spherical droplets due to surface tension. The liquid minimizes its surface area by forming a shape with the least surface area for a given volume-a sphere.
Capillary action: Surface tension plays a key role in capillary action, which is the ability of a liquid to flow in narrow spaces without external forces, such as gravity. Capillary action occurs when the adhesive forces between the liquid and a solid surface (such as glass) are stronger than the cohesive forces between the liquid molecules. This is why water rises in a thin glass tube or how plants draw water up from their roots through capillaries in their stems.
Soap bubbles: Soap bubbles are another example where surface tension is important. A soap film consists of a thin layer of water sandwiched between two layers of soap molecules. The surface tension of the water is reduced by the soap, allowing the bubble to expand and remain stable.
Applications of surface tension
Detergents and cleaning: Surface tension is directly related to the effectiveness of cleaning agents. Soaps and detergents reduce the surface tension of water, making it easier for the water to spread and penetrate surfaces, allowing for better cleaning. This is why detergents are commonly used to wash greasy surfaces, as they break the water’s surface tension and enable it to interact more effectively with the grease.
Inkjet printing: Surface tension is critical in inkjet printing technology, where tiny droplets of ink are ejected onto paper to form images or text. The surface tension of the ink must be carefully controlled to ensure that the droplets are uniform in size and do not spread excessively on the paper.
Medical devices: Surface tension is also important in the design of medical devices, such as needles and syringes. For instance, the surface tension of liquids can influence the flow of fluids through small tubes or channels in microfluidic devices, which are used in diagnostics and drug delivery.
Surface tension is a fundamental property of liquids that has a wide range of applications in nature, industry and technology. By understanding the forces that give rise to surface tension and how it can be controlled, scientists and engineers can develop new materials and processes that take advantage of this important property. Whether in cleaning products, medical devices or everyday phenomena like water droplets, surface tension plays a key role in many aspects of our daily lives.
Citation: Liang Z (2025). Surface Tension: Physics Behind Liquid Surfaces. J Phys Chem Biophys.14:437.
Received: 09-Aug-2025, Manuscript No. JPCB-24-35054; Editor assigned: 12-Aug-2025, Pre QC No. JPCB-24-35054 (PQ); Reviewed: 26-Aug-2025, QC No. JPCB-24-35054; Revised: 02-Sep-2025, Manuscript No. JPCB-24-35054 (R); Published: 09-Sep-2025 , DOI: 10.35841/2161-0398.25.15.437
Copyright: © 2025 Liang Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.