Emergency Medicine: Open Access
Open Access
ISSN: 2165-7548
ISSN: 2165-7548
Research Article - (2015) Volume 5, Issue 5
Early surgical site infection (SSI) detection could save costs and prevent morbidity. Our aim is to evaluate whether C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), leukocyte count (WBC), or high sensitivity CRP (hsCRP) measurements could effectively predict a SSI, and establish a low-cost method for early diagnosis. This prospective study was conducted at Chi-Mei Medical Center from January 2004 to December 2005. Eligible spinal surgery patients received CRP, ESR, WBC, and hsCRP measurements at pre-specified days. SSI was identified using definitions from the Centers for Disease Control and Prevention and the National Nosocomial Infection Surveillance. Eighty-five patients were enrolled. Four patients experienced SSIs (4.71%). CRP and hsCRP levels from day 2 to 14 demonstrated statistically significantly higher in SSI patients (P<0.001). A threshold CRP value of 25.4 mg/L at day 7 resulted in a sensitivity of 100% and specificity of 83.3%. A threshold CRP value of 12.05 mg/L at day 14 resulted in a sensitivity of 100% and specificity of 96.7%. ESR was a predictor of a SSI at day 14 (P<0.00001). CRP and hsCRP measurements are effective in predicting SSI. Comparing CRP values between baseline and day 2 is potentially the most cost-effective method for diagnosing post-spine surgery SSIs.
Keywords: Erythrocyte sedimentation rate; White blood cell count; High sensitivity C-reactive protein; Surgical site infection; Spine surgery
Surgical site infection (SSI) is a ubiquitous concern during the post-operative period. SSIs can prolong hospital stays and increase cost, all the while increasing morbidity and mortality [1-3]. This is particularly true with SSIs occurring after spine surgery [4-6] with costs expected to increase even further in the future due to increased co-morbidities. As such, it is imperative that spinal SSIs are discovered earlier and more accurately to allow for prompt administration of appropriate antibiotics and if necessary, surgical washout, to prevent any further damage.
Ideally, diagnosis of an SSI would require a simple, low-cost diagnostic modality. Apart from clinical monitoring, there are a number of diagnostic tools that are currently being used in practice to evaluate the presence of an SSI. These indices include elevated white blood cell counts (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and high sensitivity C-reactive protein (hsCRP). Previous studies have demonstrated that WBC and ESR have limited diagnostic potential when it comes to early diagnosis of spinal SSI [7,8]. In contrast, studies have demonstrated that CRP levels have clear diagnostic potential [7-12].
High sensitivity C-reactive protein measurements allow for lower detection thresholds for C-reactive protein. This particular lab test has had proven clinical utility in risk assessment for cardiovascular disease. However, it’s utility in diagnosing spinal SSI has not previously been investigated, although uncertainty of its importance from a clinical point-of-view still exists [13].
Although previous studies have already established the merit of serial CRP measurements in early diagnosis of SSI following spine surgery, it is not clear how this directly compares to use of ESR, WBC, or even hsCRP. Furthermore, the time course for the nadir and the zeniths of these parameters has not been established in the literature. In this study, we hope to address these concerns as well as establish a clinically relevant, cost-effective method of utilizing these parameters for early detection of post-spinal surgery SSIs.
This prospective cohort study was conducted at Chi-Mei Medical Center from January 2004 to December 2005. Patients eligible for the study had undergone a lumbar discectomy, laminectomy, or instrumented spinal fusion (the latter procedure used for treatment of spondylolisthesis) performed at a single center. Patients with postoperative fevers due to sinusitis, pharyngitis or other upper respiratory infections, urinary tract infections, deep venous thrombosis, viral syndromes, or drug reactions were excluded from the study. 85 patients (26 discectomy, 46 laminectomy, and 13 fusions with instrumental fixation) met the inclusion criteria and were included in the study. All patients were treated for lumbar spine pathologies. The study was approved by the Chi-Mei Medical Center Institutional Review Board and appropriate consents were obtained from all patients.
Surgery and data collection
All procedures were performed under general anesthesia. All patients in the study received 1 gram of cefazolin intravenously every 8 hours and 80 grams of gentamycin intravenously every 12 hours for the first 24 hours post-operatively.
Surgical management of herniated lumbar discs consisted of a posterior-approach microscopic discectomy technique. Laminectomies were similarly performed through a posterior approach. Treatment for spondylolisthesis included a decompressive laminectomy followed by a posterolateral fusion with an autologous iliac crest graft. Instrumentation was then used to stabilize the fusion.
Laboratory values for WBC, ESR, CRP, and hsCRP were determined at various time points. Measures were taken immediately before surgery and then at 2 days, 4 days, 1 week, and 2 weeks post-operatively. CRP [14] and hsCRP [15] levels were measured via nephelometry. ESR levels were determined manually by the Westergren method [16].
Infection control specialists through prospective surveillance identified sSIs. SSIs were diagnosed using definitions provided by the Centers for Disease Control and Prevention and the National Nosocomial Infection Surveillance. This involved using wound cultures, blood cultures or frank pus obtained by needle aspiration or surgical debridement. Additionally, local signs of infection, including erythema, drainage, swelling, and dehiscence were also considered. If there was a suspicion of SSI, diagnostic imaging including a non-contrast lumbar CT and an MRI with and without gadolinium was performed.
If an SSI was diagnosed, the patient was taken to the operating room for exploration and debridement of the surgical wound. Bone graft, with or without instrumentation, was allowed to remain in situ if not grossly suppurative or necrotic14 as judged by intra-operative exploration. Allograft material that was grossly infected was removed, although metallic implants were generally left in place to preserve stability and decrease risk of pseudarthrosis, which could further hinder resolution of infection [17]. Patients with suspected infection were treated with antibiotics depending on bacterial susceptibility.
Statistics
The patients were first divided into two groups, based on the presence of an SSI. The non-SSI group was then subdivided into three groups, based on the specific type of surgical procedure the patient received – discectomy, laminectomy, or instrumented fusion. These groups were compared to one another and the SSI group. All comparisons between the groups were performed using unpaired t tests or paired t tests when appropriate. A P value less than 0.05 was considered statistically significant. Threshold values were calculated by the receiver operating characteristics (ROC) curve. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for threshold values were assessed. Statistics were performed using Prism software.
During the two-year period of the study, 85 spinal surgeries performed at Chi-Mei Medical Center were included for analysis. Twenty-six patients (14 men and 12 women) with a mean age of 41 years (range: 16-69 years) had lumbar herniated discs treated by microscopic discectomy. Forty-six patients (28 men and 18 women) with a mean age of 63 (range: 36-81) years received laminectomies for treatment of spinal stenosis. Thirteen patients (10 men and 3 women) with a mean age of 56 years (range: 28-79) were treated with posterolateral bone fusion with transpedicular instrumentation for spondylolytic spondylolisthesis. The incidence of SSI was 4.71% (4/85 procedures), with two SSIs in each of the laminectomy and posterolateral fusion subgroups. There were no SSIs observed in patients receiving a microscopic discectomy.
Infection cases
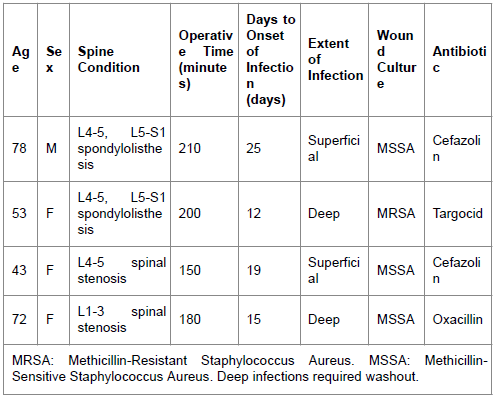
There were four total infections observed in this study. Of these four infections, two cases were observed after instrumented spinal fusion. The incidence of SSIs associated with this procedure was 15.4%. One case was a 78 year-old man who underwent L4 to S1 transpedicular instrumented fixation and posterolateral bone fusion due to L4-5 and L5-S1 spondylolytic spondylolisthesis. Wound-related pain, erythema, and local warmth were noted 25 days after surgery. The patient received local wound care and cefazolin for two weeks with symptomatic improvement. The other case was a 53 year-old woman who underwent an L4 to S1 transpedicular instrumented fixation and posterolateral bone fusion due to L4-5, L5-S1 spondylolytic spondylolisthesis as well. Pus formation, local warmth, and dehiscence were noted 12 days post-operatively. The wound culture showed oxacillin-resistant Staphylococcus aureus (MRSA). The surgical wound was explored and debrided. Afterwards, the patient was treated with intravenous Targocid (Sanofi-Aventis), a glycopeptide antibiotic that is comparable to vancomycin in terms of bacterial susceptibility [18], for 4 weeks. This resulted in resolution of the SSI.
There were two cases of SSI in patients receiving laminectomies, corresponding to an SSI incidence of 4.35% within this particular subgroup. One case was a 43 year-old woman who underwent L4-5 laminectomies due to L4-5 spinal stenosis. Wound-related pain, erythema, and local warmth were noted nineteen days after surgery. The patient received local wound treatment and cefazolin for two weeks and the wound condition improved. Another case was a 72 year-old woman who underwent L1-3 laminectomies due to L1-3 spinal stenosis. Pus formation, local warmth, and dehiscence were noted 15 days after surgery. A wound culture demonstrated the presence of oxacillin-sensitive Staphylococcus aureus (MSSA). This particular patient was treated for her SSI with surgical wound exploration and debridement, along with intravenous oxacillin for four weeks, with resolution of the SSI subsequently. Data pertaining to the four cases of infection is included in Table 1. The overall rate of SSIs requiring surgical debridement was 2.35% (2/85 patients).
Serum marker kinetics
WBC, ESR, and CRP/hsCRP all behaved differently with characteristic profiles. Although overall inflammatory profiles varied between these indices, all lab values tended to increase and form a peak, beginning around day 2 post-operatively. This peak was formed regardless of whether or not there was an infection present and the magnitude of this peak was in no way a predictor of infection.
WBC levels tended to peak at day 2 and then decline until day 4. At this point, patients with infection had observed increases in WBC levels, while WBC levels in those without infection plateaued. Despite this graph divergence, differences in WBC levels were never statistically significant within the first two weeks of post-operative serum measurements.
ESR levels tended to peak around day 4. After day 4, ESR values increased steadily in patients with infection, while ESR values decreased in patients without infection. Statistically significant differences in ESR were observed at day 14 at the earliest (P<0.0001).
CRP and hsCRP had essentially identical profiles. In patients without infection, CRP and hsCRP levels peaked at day 2, while declining consistently afterwards. In patients with infection, CRP and hsCRP levels peaked at day 4, and began to plateau. Statistically significant differences in CRP and hsCRP were observed as early as day 4 (P<0.0001). These differences continued to be statistically significant at later time-points. Of note, both CRP and hsCRP values displayed significant differences when comparing values from day 2 to values from day 14 (P<0.001 for both indices) in patients without an SSI. This difference was not observed to be significant when comparing values from day 2 to those from day 14 in patients without an SSI.
Graphs displaying post-operative kinetics for each inflammatory marker are provided in Figure 1, stratified based on procedure. Figure 2 displays inflammatory marker kinetics for stratified by infection status.
ROC analysis
ROC analysis was performed on all four serum markers. Analysis revealed that even at day 14, both ESR and WBC levels did not have statistically significant area under curve (AUC) values. For WBC values at day 14, AUC=0.7269 (p=0.1298). For ESR values at day 14, AUC=0.8278 (p=0.05694). CRP and hsCRP were mostly equivalent and thus additional analysis was focused on determining threshold CRP values.
A threshold CRP value of 25.4 mg/L (AUC=0.9242, p<0.01) at day 7 resulted in a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 100%, 83.3%, 26.7%, and 100%, respectively. A threshold value of 12.05 mg/L (AUC=0.9886, p<0.01) at day 14 resulted in a sensitivity, specificity, PPV, and NPV of 100%, 96.7%, 66.7%, and 100%, respectively. ROC curves for CRP values at day 7 and 14 have been reproduced in Figure 3.
Surgical site infection following spine surgery has a variably reported incidence generally below 8% [19-27]. Such infections often require aggressive treatment, including additional antibiotics, irrigation, and debridement, increasing health care costs and morbidity. Given the important health ramifications and costs, early and accurate detection of SSIs is necessary.
Since clinical signs, such as fever, redness, or induration, are not often observable early in the course of SSI, physicians must rely on diagnostic tests, including serum markers, imaging, and biopsy. Diagnostic imaging of such wounds generally involves magnetic resonance imaging (MRI), a fairly costly modality that would not be economically feasible to perform in all patients as a screening tool. Additionally, biopsy would have similar costs as fluoroscopic guidance would be required for accurate sampling of deeper tissues.
As serum markers are usually the easiest obtained and are the least costly, they present a veritable solution. Many previous studies have investigated the use of inflammatory serum markers, such as WBC, CRP, and ESR, in the detection of SSI after spine surgery. WBC count and ESR rates generally have been deemed to have low value in early diagnosis of SSI, which is confirmed by this study as well. A study by Thelander et al. observed that ESR levels after uncomplicated spinal operations tended to increase and remain elevated for 21 to 42 days after surgery [7]. As the median time to appreciable onset of infection is 14 days (range of 2-83 days) [28], ESR levels may have limited diagnostic value. Similarly, WBC levels can be difficult to interpret in post-operative surgical patients. A study by Kraft et al. demonstrated that after uncomplicated lumbar spine procedures, it was difficult to predict the natural course of a patient’s WBC count. As such, WBC count had low diagnostic value [8]. Given the relative inutility of WBC and ESR levels in determining SSI, other inflammatory markers, such as CRP or hsCRP, have been used. CRP has been previously demonstrated to be superior to ESR in several previous studies. Most recently, a 2008 study by Mok et al. found that using a “second rise” as an indicator of infectious complications demonstrated serial CRP monitoring as superior to and more universally applicable than serial ESR measurements [10]. Other advantages of CRP include that it is directly measurable and is not grossly affected by concurrent pathologies other than liver failure [29]. Although there have been many publications concerning the use of CRP as a screening tool, there have not been any previously published studies that have investigated the use of hsCRP. High-sensitivity CRP is capable of measuring very low levels of CRP and has increased accuracy at these low levels [30].
Our study agreed with previous studies that asserted the superiority of serial CRP monitoring in diagnosing SSIs following spine surgery. We specifically found that CRP levels at 7 days and 14 days post-spinal surgery provided the best diagnostic indicator of SSI. At these time points, average CRP values within the infection group demonstrated a statistically significant increase over average values in the non-infection group. Furthermore, we found that in patients with infection, CRP values tended to plateau without significantly changing between days 2 and 14. This was in contrast to patients who did not have SSIs, who had significant decreases in CRP values from day 2 to day 14, reflecting decreases in inflammation. As expected, hsCRP values mimicked CRP values, with similar significant differences observed at the same time points.
In comparison, ESR values only showed statistically significant differences between infection and non-infection groups at day 14. A similar significant difference was not observed with serial WBC measurements, indicating its lack of diagnostic utility contrary to popular belief.
To further establish the clinical utility of serial CRP values in diagnosing SSI post-spine surgery, threshold values were determined. At day 7, a cutoff value of 25.4 mg/L has a sensitivity of 100% and a specificity of 83%. At day 14, a cutoff value of 12.05 mg/L had a sensitivity of 100% and a specificity of 97%. This sensitivity and specificity was greater than respective values observed in previous studies [10,11]. Furthermore, CRP values have clinical utility when comparing measurements from day 2 to those from day 14. As mentioned previously, patients with infection had continued elevation of CRP values from day 2 thru day 14, a finding not observed in patients without infection. This finding opens up the possibility of using this particular comparison as a diagnostic tool. Our data further suggests that hsCRP values should never be utilized in diagnosis of spinal SSIs, as the added benefit of a lower detection threshold is not required for the range of values observed in infection cases.
Given these findings, it is our recommendation that patients recovering from spinal surgery should be monitored with only CRP tests during the post-operative period to assess their inflammatory status and acute phase response. We suggest that patients should have CRP levels measured on day 2 and day 14. These values should then be compared to one another, with the expectation that a persistent CRP elevation indicates SSI. Additionally, the day 14 CRP value could be utilized as a diagnostic marker in and of itself. At day 14, CRP values greater than 12.05 mg/dl would suggest infection, as mentioned earlier. Lastly, WBC, ESR, and hsCRP tests are not recommended as such tests result in increased cost without increased diagnostic utility.
There are several limitations to this study. Firstly, and most importantly, the number of patients enrolled in this study is small compared to previously published studies. This resulted in fewer patients in our infected cohort, resulting in a highly variable average lab values. Secondly, we did not gather patient information pertaining to WBC differentials, which is commonly acquired in clinical practice at low cost. Further investigation into the specific differential leukocyte breakdown could have resulted in a more powerful predictor of infection status. Such a finding would have discredited our conclusion that WBC count does not have diagnostic utility in determining the presence of a spinal SSI. In conclusion, this study demonstrates that CRP is a more effective predictor of SSI in patients with spine surgery compared to WBC or ESR, confirming previous studies. Furthermore, it appears that there is no significant difference in terms of predicting post-lumbar surgery SSIs between CRP and hsCRP. The kinetics of the CRP and hsCRP tests are such that the infection group diverges from non-infection starting day 4, which persists to 2 weeks post-operatively. The threshold CRP values that proved to be the most robust in terms of ROC analysis are 25.4 mg/L at day 7 and 12.05 mg/L at day 14. A proposed strategy based on the serum kinetics of CRP would be to obtain a baseline CRP value at day 2 and compare it with the value 2 weeks post-operatively to determine whether an SSI exists or not. This strategy does not require the use of additional tests, including ESR, WBC, or hsCRP, resulting in decreased costs.
This study was supported by Grant CMFHR9301 from the Chi Mei Medical Center. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.