Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Research Article - (2016) Volume 3, Issue 4
Black spot disease has become a serious constraint for successful cultivation of rose crop in Pakistan and rest of the world. The current research was conducted to identify the causal pathogen and respective disease severity in 4 districts of province Punjab, Pakistan. The most prominent symptoms of disease included formation of purple to black spot (5-15 mm) with/without yellow margins especially on older leaves. Mortality of infected plants was also observed in severely infected plants. It was observed that Diplocarpon rosae was the casual organism of this disease. The greenhouse experiment revealed variation in pathogenicity of different pathogenic strains.
<
Keywords: Black spot; Diplocarpon rosae; Rose
Rose (Rosa sp.) belongs to family Rosaceae. There are over 200 species and more than 18000 cultivars of roses [1]. There is a shifting tendency towards sourcing floricultural products from developing countries like Bangladesh, India and Pakistan [2]. This is due to the increasing labour and energy costs in Europe. This trend has built up a pressure on the successful cultivation of healthy rose plants in the developing countries, like Pakistan. Pakistan being an agricultural country with diverse agro climatic zones has a huge potential for the cultivation of roses [3,4]. Rose is an economically important horticulture crop cultivated throughout the world and is generally referred as king of flowers [5]. It is also cultivated because of its medicinal importance. Various parts of rose plants are being used in different types of medicinal formulations. Its cultivation is performed in both open fields and protected greenhouses. Rose cultivation is increasing in Pakistan because of its ornamental and medicinal values [3,6-7]. Roses provide high revenue to farmers and increase employment opportunities [8]. In case of Pakistan, area under flowering crops is estimated more than 17000 acres [9]. The increased utilization of cut flowers in hotels, restaurants and banquet halls business has resulted due to increased living standard of people. Nevertheless, a big gap between demand and supply of flowers and relevant products still exists [10].
Rose crop is subjected to a number of insect pest and fungal diseases. The management of rose diseases requires frequent use of fungicides that adversely affect the environment and raise costs of production [11]. Diseases are an important reason for losses in agricultural crop commodities. It is estimated that world faces nearly 13% losses in agriculture produce because of plant diseases caused by a number of pathogens [12]. More than 80% plants diseases are caused by nasty fungal pathogenic microbes. Therefore fungal diseases cause a severe reduction in production and subsequently lower economic return to grower [10]. Leaf spot diseases caused by various fungi are a common pathological constraint of a number of plant species. Among different leaf spot diseases, black spot disease of rose holds an important place [13]. This fungal disease is caused by Diplocarpon rosae, and prevails throughout the world [14,15]. Almost all rose varieties are susceptible to black spot disease [16]. D. rosae causes formation of purple to black spots on leaves and shoot of infected rose plants (Figure 1). The disease symptoms first appear on older leaves present on lower area of plants, later the symptoms spreads upwards. The spots may be round or may have asymmetrical or feathery ends. The infected area of leaves turns yellow around the spots resulting in premature leaf fall. Severe infection can result in complete defoliation and dieback of infected plants. Black spots disease reduces the number of flowers, marketable value of flowers, plant vigour and the life of plant [17]. Occasionally symptoms may be observed not only on the leaves of the plants but also on petals (red spots, deformation), leaf petioles, fruit and shoots [13]. The infected plants have unattractive appearance [18]. The name Marssonina rosae is applied for the imperfect stage of the fungus while the perfect stage, D. rosae is rarely observed [19]. D. rosae may be cultured on artificial media; growth on media is very slow [20]. D. rosae may be stored at -20°C or -80°C on foliage of rose plant or as a suspension of conidia [21]. The optimum temperature for development of mycelium is 21°C. Since, leaves of rose plants raised in glasshouses present in the temperate zones may be kept dry; therefore these plants are not severely infected by black spot disease [13,19]. Different pathotypes of D. rosae have been illustrated using polyconidial [20] and monoconidial [21,22] isolates. Furthermore, different morphotypes have also been observed [23]. The scientists have observed genetic differences with different techniques and results indicate the existence of two main clusters of strains [24].
Field survey of commercial rose farms in Punjab province and collection of virulent isolates of Diplocarpon rosae
A field survey was conducted during spring season of 2012 and 2013. On the whole 40 rose farms situated at different locations scattered over 4 districts of Punjab were surveyed. Some district of Punjab province viz: Lahore, Chakwal, Rawalpindi and Kasur were selected for the survey of rose cultivated area, with particular focus on disease status and for collection of black spot infected leaf samples. These districts were selected because of their importance for rose cultivation. Moreover, these districts are important market area for rose related products comprising potted and bare rooted plants, cut flowers, rose petals, rose water and gulqand. In case of district Lahore, rose farms located near Sagian village, Sundar village and farms present in University of the Punjab, were surveyed. In case of district Kasur, Pattoki, the original and well-known growing area of roses was surveyed. However, some other locations of Kasur including Rao Khanwala and Raiwind were also surveyed. Tehsil of district Chakwal (Kalar Kahar) and its surroundings were also surveyed to evaluate the incidence and severity of the black spot disease. In case of district Rawalpindi, rose farms present in village Doltala, Chakbeli, Dhamial and Chakri were also included in this survey. Only those rose farms were evaluated where crop was cultivated on more than 1 kanal area. Moreover these farms were situated at least 3 km away from each other. Plant were evaluated and sampling was performed following hierarchical sampling approach involving 15 plants from 5 random locations across a diagonal in each farm [25]. Geographical location, altitude and climate of surveyed districts are shown in Table 1.
| Name of District | Altitude (ft) | Climate |
|---|---|---|
| Lahore | 706 | Semi-Arid |
| Kasur | 662 | Semi-Arid |
| Chakwal | 1623 | Semi-Arid |
| Rawalpindi | 1633 | Humid Subtropical |
Table 1: Altitude and climatic conditions of districts surveyed.
Whereas, symbolic representation of disease severity scales is exhibited in Figure 2. During the surveys, disease attributes viz: percentage of disease incidence, disease index and mortality of rose plants infected with black spot disease were assessed. Infected leaf samples were collected for isolation, purification and mass culturing of pathogenic isolates. Furthermore, rhizospheric soil samples from some healthy rose plants were collected.
Estimation of disease incidence (%), disease index (%) and mortality percentage of black spot disease
For assessment of disease incidence, disease index and mortality percentage, 3 plants from an open quadrate (5 × 5 ft) were randomly selected. Quadrate was randomly thrown near every corner and centre of the field by considering its geometrical lay out.
Disease incidence (%) was calculated according to formula described by Iqbal [26]. as under:
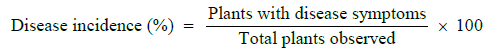
Mortality percentage of rose plants infected with black spot disease was measured as under:
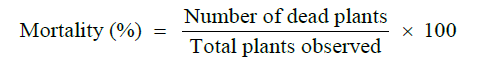
The black spot symptoms were divided into 6 classes of disease severity (DS) according to scale proposed by Gachomo (Table 2) [27].
| Class/scale | Disease Severity | Symptoms Description |
|---|---|---|
| 1 | 0% | Leaflet with no black spot symptoms |
| 2 | 1-20% | Leaflet with black spots having size less than 5 mm. No yellowing of leaflet and no premature defoliation |
| 3 | 21-40% | Leaflet with black spots having less than 5 mm size along with yellowing and/or defoliation symptoms |
| 4 | 41-60% | Leaflet with black spots having 5-10 mm size with no yellowing and/or defoliation symptoms |
| 5 | 61-80% | Leaflets with black spots of 5-10 mm along with yellowing and/or defoliation symptoms |
| 6 | 81-100% | Leaflets with black spots of more than 10 mm along with yellowing and/or defoliation symptoms |
Table 2: Disease severity rating scale of black spot of rose.
From disease severity, value of disease index (DI) was calculated using following equation as described by Palmer et al. [20].
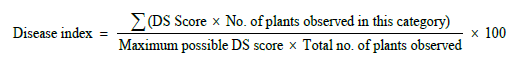
Infected rose leaves showing typical symptoms of black spot disease viz: (showing round to irregular black spots on upper leaf surface surrounded by yellow chlorotic tissues) were removed from plants and stored individually in sterilized polythene bags. These polythene bags were placed in an ice cooler and transported to the laboratory of Institute of Agricultural Sciences, where they were stored at 4°C for further studies.
Isolation and identification of pathogenic isolates
The pathogen isolates were isolated from black spot disease infected leaves of rose plants growing in commercial rose farms from different districts of Punjab province. Three leaves were randomly selected from each farm to isolate pathogen. For isolation of pathogen, 3-4 mm sections from infected parts of pre washed rose leaves were surface sterilized with 1.5% sodium hypochlorite solution for 60-90 s. These leaf sections were provided with several washings with distilled sterilized water and dried in laminar air flow cabinet. Afterwards these leaf samples were inoculated on pre-autoclaved, cool Potato Dextrose Agar (PDA) medium plates. These petri plates were incubated for 15 days at 20°C under cool-white fluorescent lamps with a 15 h photoperiod. Spores developed from leaf pieces were suspended in sterile distilled water, spread on 2% water agar, and incubated as described above for 24 h. Pure culture of pathogenic isolates was acquired through subsequent sub-culturing on freshly prepared PDA medium plates. Inoculated plates were once again incubated for 30 days at 20°C under cool-white fluorescent lamps with a 15 h photoperiod. The resulting pathogen isolates were given codes from DR1 to DR120. Isolates of D. rosae were identified based on morphological features viz: colony color, shape of hyphae, color and shape of conidia [28,29].
Screening of the most virulent pathogenic isolate of Diplocarpon rosae
A total of 120 Diplocarpon rosae isolates were obtained from infected leaves of rose plants. For screening of the most virulent isolate, response of rose plants against these isolates of D. rosae was examined. The most virulent pathogenic isolate was selected for further research work.
Preparation of fungal inoculums
Pathogen inoculum was prepared by growing the fungus on PDA plates. For that purpose, isolated pathogenic fungal cultures were sub-cultured on PDA medium plates. These inoculated plates were incubated at room temperature. After 30 days of incubation, conidia were harvested. For that purpose, conidia, present in petri plates, were rubbed gently with glass rod and dislodged in 10 mL distilled sterile water. The pure culture of pathogenic isolates was acquired through subsequent sub-culturing on freshly prepared PDA medium plates. The pure conidial suspension was then passed through two layers of muslin cloth and final concentration of spore suspension was adjusted up to 5 × 105 spore/mL by using haemacytometer.
Plant propagation
Plants of Rosa bourboniana var ‘Gruss a Teplitz’ were grown under greenhouse conditions in Rose Farm of University of the Punjab, Lahore. Uniformly sized cuttings of rose plants having 3-4 nodes were disinfected in a 0.2% (w/v) solution of Captan 50% WP to maintain sterilized conditions. These treated cuttings were raised independently in sterilized 16 kg soil medium present in 35.5 cm clay pots. Physicochemical analysis of soil revealed that it was sandy loam (in texture) having organic matter 0.72%, pH 7.6, electrical conductivity 1.66 dS m-1, nitrogen 0.035%, available phosphorus 6.5 mg.kg-1 and available potassium 100 mg.kg-1. The quantity of micronutrient including boron, manganese, iron, copper and zinc were 0.95, 20, 10, 2 and 1.2 mg.kg-1 of soil respectively. All plants were watered every second day with equal quantity of sterilized distilled water. The plants received no additional fertilizer, pest and disease control measures.
Greenhouse experiment
Eight months old potted rose plants were tagged and tested individually against all isolates of D. rosae. Just before spray, soil surface of potted plants was covered with plastic sheet to avoid the unwanted fall of pathogenic inoculum on soil surface. With the help of hand spray bottle, foliage of potted rose plants was sprayed with respective pathogenic isolates, on allotted rose plants according to the experimental design whereas, negative control received just spray of distilled sterilized water. Afterward polythene sheet was removed from soil surface. Treated rose plants were placed under translucent polythene tunnel to entrap humidity which aids pathogen attack. Each pot was provided equal quantity of distilled sterilized water every second day. Pathogen isolate which exhibited maximum disease index after 20 days post inoculation (dpi) was termed as most virulent isolate (MVI) and used for downstream trials (Figure 3).
Statistical analysis
All relevant data were statistically analyzed by performing ANOVA and Duncan’s multiple range test (DMRT) using computer aided program DSAASTAT (Onofri Italy).
Field survey of commercial rose farms in Punjab province and collection of virulent isolates of Diplocarpon rosae
A survey was carried out to assess the disease incidence, disease index and mortality % of black spot disease of roses from different farms present in Lahore, Kasur, Chakwal and Rawalpindi. Rose plants infected by black spot disease were observed in all inspected rose farms. Preliminary disease symptoms were development of minute black to purple brown spots on fully opened older leaves of the infected plants. Premature fall of severely infected leaves was also noticed. Moreover, dead rose plants exhibiting symptoms of black spot disease on their canes were also observed. In general, severely infected plants were defoliated and exhibited symptoms of black spots on their shoots. A total of 40 rose farms in Lahore, Kasur, Chakwal and Rawalpindi districts were surveyed. From surveyed farms 600 rose plants were selected randomly and evaluated for disease attributes including disease incidence, disease index and mortality percentage. The values of disease attributes were significantly different in some surveyed farms present in different districts of Punjab (Tables 3-5).
| Rose Farm Visited | Disease Incidence (%) | |||
|---|---|---|---|---|
| Lahore | Kasur | Chakwal | Rawalpindi | |
| Rose Farm 1 | 87.33 ± 07.15a | 64.06 ± 05.11b | 54.76 ± 04.78c | 83.26 ± 07.13a |
| Rose Farm 2 | 25.6 ± 03.58g | 51.9 ± 04.45cd | 18.2 ± 01.76g | 34.2 ± 03.18e |
| Rose Farm 3 | 52.96 ± 04.72ef | 20.36 ± 01.84f | 48.96 ± 04.47d | 54.7 ± 04.27c |
| Rose Farm 4 | 69.1 ±05.31b | 44.26 ± 04.34e | 50.4 ± 04.05cd | 71.3 ± 06.65b |
| Rose Farm 5 | 62.36 ± 04.45c | 43.13 ± 03.21e | 43.26 ± 04.16e | 62.3 ± 05.71b |
| Rose Farm 6 | 46.66 ± 03.87f | 69.56 ± 05.25a | 60 ± 05.84b | 54.8 ± 04.59c |
| Rose Farm 7 | 53.96 ± 04.47de | 20.66 ± 01.87f | 60.4 ± 05.97b | 51.7 ± 05.48d |
| Rose Farm 8 | 58.56 ± 04.09c-e | 46.6 ± 03.07de | 42.03 ± 03.06e | 59.5 ± 05.63bc |
| Rose Farm 9 | 22.93 ± 02.57g | 56.83 ± 04.65bc | 24.82 ± 02.58f | 29.9 ± 02.09e |
| Rose Farm 10 | 60.1 ± 05.84cd | 57.8 ± 04.45bc | 68.6 ± 05.57a | 68.5 ± 04.25b |
Table 3: Means for black spot disease incidence (%) on rose farm present in different districts of the Punjab.
| Rose Farm visited | Disease Index (%) | |||
|---|---|---|---|---|
| Lahore | Kasur | Chakwal | Rawalpindi | |
| Rose Farm 1 | 52.12 ± 04.17b | 39.72 ± 03.5d | 19.65 ± 01.9e | 46.6 ± 03.de |
| Rose Farm 2 | 39.02 ± 03.95d | 21.87 ± 01.e | 38.94 ± 03.7d | 20.36 ± 01.5f |
| Rose Farm 3 | 66.53 ±05.a | 68.33 ± 05.9a | 36.75 ± 02.d | 44.26 ± 04.e |
| Rose Farm 4 | 53.55 ± 04.27b | 54.17 ± 04.65b | 35.20 ± 02.17d | 65.06 ± 05.b |
| Rose Farm 5 | 47.34 ± 03.95c | 48.15 ± 03.bc | 51.97 ± 04.9bc | 51.9 ± 04.5cd |
| Rose Farm 6 | 59.05 ± 05.b | 17.2 ± 01.9e | 46.05± 03.bc | 20.66 ± 01.f |
| Rose Farm 7 | 21.8 ± 02.3e | 39.86 ± 03.57d | 18.6± 01.17e | 71.56± 06.a |
| Rose Farm 8 | 37.87 ± 02.75d | 52.19 ± 04.b | 65.32 ± 05.02a | 43.13 ± 03.97e |
| Rose Farm 9 | 38.66 ± 03.9d | 55.61 ± 04.9b | 45.32 ± 04.05bc | 55.83 ± 04.7bc |
| Rose Farm 10 | 22.13 ± 02.47e | 43.10 ± 03.8c | 42.7 ± 03.06c | 59.8 ± 04.bc |
| Mean values sharing similar letters in a column are not significantly different (p=0.05) | ||||
Table 4: Means for disease index (%) of black spot disease on different rose farms in selected districts of Punjab province.
| Rose Farm Visited | Mortality (%) | |||
|---|---|---|---|---|
| Lahore | Kasur | Chakwal | Rawalpindi | |
| Rose Farm 1 | 1.80 ± 0.15c | 1.12 ± 0.07d | 1.8 ± 0.07b | 1.07 ± 0.04g |
| Rose Farm 2 | 2.02 ± 0.13b | 1.13 ± 0.08e | 1.58 ± 0.12b | 1.9 ± 0.05cd |
| Rose Farm 3 | 1.42 ± 0.12d | 1.6 ± 0.12d | 1.67 ± 0.13b | 1.37 ± 0.09fg |
| Rose Farm 4 | 1.39 ± 0.11d | 1.15 ± 0.09e | 1.64 ± 0.11b | 1.7 ± 0.07de |
| Rose Farm 5 | 1.43 ± 0.12d | 2.7 ± 0.16a | 1.65 ± 0.08b | 2.89 ± 0.21a |
| Rose Farm 6 | 1.38 ± 0.1d | 2.01 ± 0.19bc | 1.26 ± 0.05c | 2.13 ± 0.14bc |
| Rose Farm 7 | 2.4 ± 0.19a | 1.8 ± 0.11cd | 2.1 ± 0.15a | 2.26 ± 0.21b |
| Rose Farm 8 | 2.2 ± 0.18b | 1.6 ± 0.16d | 2.0 ± 0.16a | 2.08 ± 0.17bc |
| Rose Farm 9 | 1.09 ± 0.09e | 1.63 ± 0.12d | 1.03 ± 0.06c | 1.46 ± 0.09ef |
| Rose Farm 10 | 1.14 ± 0.11e | 2.25 ± 0.2b | 1.13 ± 0.03c | 1.14 ± 0.05g |
| Values provided are mean of two year experiment. Values sharing similar letters in same column are not significantly different (p=0.05) | ||||
Table 5: Means for mortality (%) caused by black spot disease of rose on rose farms in selected districts of Punjab province.
Estimation of disease incidence (%), disease index (%) and mortality percentage of black spot disease
The maximum means for disease incidence (56%) was recorded in case of rose farms present in district Kasur followed by 53, 46 and 43.6% observed in district Rawalpindi, Lahore and Chakwal respectively (Figure 3a). On the other hand maximum disease index (47.1%) was recorded in district Rawalpindi followed by district Kasur (44%), district Lahore (43.8%) and district Chakwal (40%) (Figure 3b). Similarly, maximum value for disease mortality (1.8%) was recorded in rose farms present in district Rawalpindi followed by district Kasur (1.69%), district Lahore (1.62%) and district Chakwal (1.58%) (Figure 3c).
During this survey infected leaves showing typical symptoms of black spot disease were brought in the laboratory and 120 pathogenic isolates were obtained. Locations from where these isolates were obtained are presented in Table 6.
| D. rosae isolate | Location | D. rosae Isolate | Location |
|---|---|---|---|
| DR1-DR6 | Gahlan, Kasur | DR61-DR63 | Bherpur, Chakwal |
| DR7-DR9 | Sajhowal, Kasur | DR64-DR69 | WatooWala, Chakwal |
| DR10-DR12 | Daoki, Kasur | DR70-72 | Bulkasar, Chakwal |
| DR13-DR15 | Srayser, Kasur | DR73-DR75 | Dhudial, Chakwal |
| DR16-18 | Nizampura, Kasur | DR76-DR81 | KallarKahar, Chakwal |
| DR19-DR21 | Jagowala, Kasur | DR82-DR84 | Bhaun, Chakwal |
| DR22-DR24 | Theeng, Kasur | DR85-DR87 | Rupowal, Chakwal |
| DR25-DR27 | Raja jang, Kasur | DR88-90 | Dhodha, Chakwal |
| DR28-DR30 | Raokhanwala, Kasur | DR91-DR93 | Doltala, Rawalpindi |
| DR31-DR33 | Hanjarwal, Lahore | DR94-DR96 | Jatli, Rawalpindi |
| DR34-DR36 | Shahdra, Lahore | DR97-DR102 | ChakBeli, Rawalpindi |
| DR37-DR39 | Chungi-Amar-Sdhu, Lahore | DR103-DR105 | Tarnol, Rawalpindi |
| DR40-DR42 | Thokar, Lahore | DR106-DR108 | Gujar Khan, Rawalpindi |
| DR43-DR45 | Sundar, Lahore | DR109-111 | Rawat, Rawalpindi |
| DR46-DR48 | University of the Punjab,Lahore | DR112-114 | Choor, Rawalpindi |
| DR49-57 | Sagian, Lahore | DR115-DR117 | Shamshabad, Rawalpindi |
| DR58-DR60 | Bund Road, Lahore | DR118-DR120 | Nathiabania, Rawalpindi |
Table 6: Detail of pathogen isolates isolated from diseased plants from different locations.
Pathogenic isolates including DR1 to DR30 were isolated from different rose farms present in district Kasur while pathogen isolates DR31 to DR60 were isolated from district Lahore. Similarly isolate viz: DR61 to DR90 were isolated from district Rawalpindi. Whereas, fungal isolates DR91 to DR120 were isolated from rose farms present in district Kasur.
Isolation and identification of pathogenic isolates
The fungi isolated from diseased samples obtained from different localities were identified using microscopic characters and keys. Generally, fungus growth in PDA medium was very slow. Fungal colonies grown on petri plates appeared light grey in colour. The top and periphery of the fungal colony showed white mycelium however base of the colony were yellowish in colour. The hyphae were branched and septate. The apothecium of fungal isolates was almost round to disc shaped. Whereas ascocarp were cup shaped. Conidia were present in acervuli, and it contained a flat round mass of hyphae. The conidia having 15-25 × 5-7 μm size were hyaline, elliptical, separated into two parts by a septum. The fungal isolates were identified as Diplocarpon rosae (Figure 2).
Screening of most virulent pathogenic isolate of Diplocarpon rosae
Greenhouse experiment: This experiment was performed to screen most aggressive isolate of Diplocarpon rosae. The pathogenic isolates of D. rosae isolated during survey were tested on Rosa bourboniana var ‘Gruss an Teplitz’ for their virulence. Statistical analysis showed that rose plants inoculated with D. rosae revealed highly significant (p=0.05) interactions in case of some isolates. The leaves of rose plants exhibited symptoms of black spot disease within 12 to 15 days after pathogen inoculation. General symptoms observed included formation of purple to black spot (5-15 mm) with/without yellow margins on older leaves. However, in case of DR19 young and old leaves of plants showed characteristic symptoms of black spot disease (Figure 2). Shoots of some severely infected plants were also covered by black spots. Premature defoliation was also observed in case of rose plants severely infected by black spot disease.
All fungal isolates were found pathogenic and resulted in development of disease symptoms upto varying degree of significance (p=0.05) as compared to control. It was found that DR19 which was isolated from district Kasur was recorded as the most virulent pathogenic isolate. This isolate showed the average disease index of 86.6% which was significantly highest (p=0.05) among all pathogen isolates obtained from surveyed districts. In case of district Lahore DR32 was least aggressive isolate and could reveal disease index of 24.2%. DR38 isolate obtained from district Lahore was most aggressive isolate for this district and plants inoculated by DR38 showed 61% disease index (Table 7). DR6 was least virulent among all isolates collected from district Kasur. DR6 applied rose plants showed 23.3% disease index (Table 7).
| Pathogen Isolates | Disease Index | Pathogen Isolates | Disease Index | Pathogen Isolates | Disease Index |
|---|---|---|---|---|---|
| DR1 | 58.33±4.1e-g | DR51 | 24.6 ± 1.6vw | DR81 | 42.6 ± 3.2kl |
| DR2 | 45.3 ± 2.6k | DR52 | 53.6 ± 3.7g-j | DR82 | 32.3 ± 1.4r-t |
| DR3 | 45.6± 4.1k | DR53 | 56.6 ± 5.1f-j | DR83 | 36.6 ± 2.7o-r |
| DR4 | 25± 1.8vw | DR54 | 46.3 ± 3.3k | DR84 | 25.6 ± 1.9vw |
| DR5 | 55± 4.9g-j | DR55 | 23.6 ± 1.4vw | DR85 | 64.6 ± 64.4b-d |
| DR6 | 23.3 ± 3.7vw | DR56 | 53.6 ± 5.9g-j | DR86 | 42 ±2.9k-n |
| DR7 | 52.3 ± 4.3j | DR57 | 25± 1.1vw | DR87 | 58 ±3.7e-h |
| DR8 | 26.3 ± 1.3vw | DR58 | 27 ±1.5u-w | DR88 | 34.3 ± 3.1p-s |
| DR9 | 25 ± 0.8vw | DR59 | 44.6 ± 3.6kl | DR89 | 57.3 ± 4.1e-i |
| DR10 | 64 ± 5.8b-d | DR60 | 35.3 ± 3p-s | DR90 | 37.3 ± 3.1n-q |
| DR11 | 65± 4.42b-d | DR61 | 31.3 ± 2.3s-u | DR91 | 44.3 ± 2.3kl |
| DR12 | 24.6 ± 6.7vw | DR62 | 53.6 ± 4.6g-j | DR92 | 35.3 ± 2.5p-s |
| DR13 | 57± 2.9e-j | DR63 | 63.3 ± 3.3b-d | DR93 | 25 ± 0.7vw |
| DR14 | 45.6 ± 2.7k | DR64 | 52.3 ± 4.2j | DR94 | 44.3 ± 2.9kl |
| DR15 | 25.3 ± 1.9vw | DR65 | 60.3 ± 5.1d-f | DR95 | 44 ± 2.8kl |
| DR16 | 35.6 ± 1.7o-s | DR66 | 45.3 ± 3.3k | DR96 | 43.3 ± 3.3kl |
| DR17 | 24.3 ± 2.3vw | DR67 | 36.6 ± 2.7o-r | DR97 | 63.6 ± 3.9b-d |
| DR18 | 53 ±5.1ij | DR68 | 56.3 ± 3.3f-j | DR98 | 55 ± 4.7g-j |
| DR19 | 86.6 ± 6.7a | DR69 | 63.6 ± 67b-d | DR99 | 32.3 ± 2.3r-t |
| DR20 | 24.6 ± 1.7vw | DR70 | 22.3 ± 33vw | DR100 | 57.3 ± 2.8e-i |
| DR21 | 31.6 ± 2.7s-u | DR71 | 35.3 ± 33p-s | DR101 | 64.6 ± 3.6b-d |
| DR22 | 25.6 ± 1.6vw | DR72 | 22.3 ± 1.3w | DR102 | 42.3 ± 3.3k-m |
| DR23 | 37.6 ± 2.7m-p | DR73 | 44 ±2.8kl | DR103 | 67.6 ± 4.7b |
| DR24 | 58.3 ± 3.3e-g | DR74 | 64.6 ± 6.7b-d | DR104 | 65.3 ± 4.3bc |
| DR25 | 34.3 ± 1.3p-s | DR75 | 45± 3.9kl | DR105 | 20.6 ± 0.6vw |
| DR26 | 54.3 ± 4.3g-j | DR76 | 43 ±3.5kl | DR106 | 65.6 ± 6.7bc |
| DR27 | 52.6 ± 4.6ij | DR77 | 55± 4.4g-j | DR107 | 35 ± 2.9p-s |
| DR28 | 28± 1.9t-v | DR78 | 31.3 ± 3.3s-u | DR108 | 53.3 ± 4.3h-j |
| DR29 | 34.3 ± 2.5p-s | DR79 | 65.6 ± 6.7b-d | DR109 | 44.3 ± 3.9kl |
| DR30 | 34.3 ± 3.1p-s | DR80 | 25.6 ± 1.6vw | DR110 | 56.6 ± 4.1f-j |
| DR31 | 25± 1.7vw | DR91 | 31.3 ± 2.1s-u | DR111 | 44.3 ± 3.2kl |
| DR32 | 24.2± 1.9vw | DR92 | 40.3 ± 2.3l-o | DR112 | 55 ± 3.8g-j |
| DR33 | 25.6 ± 1.9vw | DR93 | 46.3 ± 2.3k | DR113 | 46 ± 3.2k |
| DR34 | 42.6 ± 3.6kl | DR94 | 31.3 ± 2.9s-u | DR114 | 33.6 ± 1.6p-s |
| DR35 | 57± 4.8e-j | DR95 | 25.3 ± 1.2vw | DR115 | 69.3 ± 3.3b |
| DR36 | 46.3 ± 3.3k | DR96 | 63.3 ± 5.3b-d | DR116 | 24.3 ± 1.3vw |
| DR37 | 43 ±2.7kl | DR97 | 22.6 ± 1.7w | DR117 | 24.6 ± 1.7vw |
| DR38 | 61.6 ± 5.6c-e | DR98 | 24.6 ± 0.6vw | DR118 | 31.6 ± 2.7s-u |
| DR39 | 54.6 ± 4.7g-j | DR79 | 52.6 ± 3.7ij | DR119 | 26 ± 0.9vw |
| DR40 | 32.6 ± 1.7q-t | DR80 | 63.3 ± 4.3b-d | DR120 | 63.3 ± 5.3b-d |
| Negative Control | 0 | ||||
| Significance of data were calculated through DMRT at (p=0.05) and mentioned by alphabetic letters | |||||
Table 7: Relative disease index caused by different isolates of Diplocarpon rosae.
In this interaction, the least virulent isolates obtained from district Chakwal was DR70 while most virulent isolate obtained from this district was DR79, plants treated by these isolates exhibited 22.3 and 65.6% disease index respectively. In case of isolates obtained from district Rawalpindi, least virulent isolate DR105 treated plants showed 20% disease index whereas most virulent isolate in this district (DR115) demonstrated 69.3% disease index. Therefore, DR19 isolate which showed maximum value for disease index was declared most virulent isolate of surveyed farms and was selected for further investigation (Table 7).
Application of fungicides is a most common strategy for management of fungal pathogens since a long time [30]. It is observed that with the passage of time, many fungal pathogens have developed acquired resistance against these chemicals. Moreover, these pesticides have harmful effects on human being and environment [31,32]. Keeping in view the detrimental effects of these fungicides, it becomes necessary to find out some safe strategy to manage diseases. The inherent plant resistance is one of the efficient means to control disease. This innate resistance may be improved by the inoculation of some biological inducers such as bacillus and pseudomonas strains. Our present studies are advancement in the similar way. Our study involves enhancement of innate resistance factors of rose plants by treating with rhizospheric bacterial strains.
Since D. rosae is such an overwhelming pathogen of roses, it is vital to try to manage it by some useful and economical method. During our current research, considerable difference in pathogenic potential was observed between the 120 D. rosae isolates tested, with D. rosae DR19 proving to be the most aggressive. Our findings are similar to the results revealed by Debener et al. [21] who observed different physiological races of this pathogen. Gachomo [27] also observed variation in virulence of different isolates of D. rosae. Some other researchers also found variation in susceptibility of rose plants to different isolates of D. rosae [33,34]. The difference in virulence of different fungal isolates may be due to variations in their genetic makeup [35,36]. This genetic difference may trigger formation of unlike proteins resulting different level of susceptibility. Therefore, different isolates were capable to exhibit variability in their virulence [3]. However, the cultivars which have sufficient inherent resistance generally evade the attack of different species of pathogens [37,38]. The logic for this kind of broad-spectrum resistance is that different factors of plant resistance help these cultivars to resist against pathogen [39,40].
The Diplocarpon rosae causing black spot disease of roses is a serious problem for rose cultivation. This disease prevails in all rose farms of the Pakistan. It is therefore recommended to apply proper disease management strategies to reduce the damage and jeopardy of the disease. Genetic manipulation of rose varieties and application of appropriate strategies may enhance commercial value of this crop.