
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research - (2022)Volume 13, Issue 4
Background: Outcomes are poorest in severe and critical COVID-19 pneumonia. We share our experience with the use of interleukin-6 receptor inhibitor, tocilizumab in the above patient group when given along with steroids.
Methods: In this retrospective observational study, all severe and critical COVID-19 patients, who got admitted to the intensive care unit and subsequently received tocilizumab were included. Patients who worsened clinically or had no change in oxygen requirement even after 24 hrs of receiving intravenous methylprednisolone at a dose of 1-2 mg/kg/day had received a maximum total dose of 800 mg of intravenous tocilizumab. The day 28 all-cause mortality and progression to mechanical ventilation were the primary outcome measures. Clinical improvement, trends in oxygen requirements and inflammatory markers along with secondary infections rates were also noted.
Results: A total of 51 patients who did not show clinical improvement even after 24 hours of intravenous steroids had received tocilizumab. Following this, a significant decrease in oxygen requirement and clinical progression were observed by day 7. Twelve (24.5%) of the 49 patients who were on conventional or non-invasive oxygen support progressed to mechanical ventilation and the day 28 all-cause mortality was 10/51(19.6%). Irrespective of the outcome, CRP showed a significant decrease after the drug administration. However, rest of the inflammatory markers became significantly deranged in those with poorer outcome (day 3 ferritin (p=0.026), day 7 (p=0.041) ddimer and NLR (p=0.044)) as compared to patients who were alive at day 28. Life-threatening infections occurred in 19.6% of the 51 patients and were significantly more in those who expired on or before day 28. (70% vs. 7.3%, p<0.001).
Conclusion: Early and timely administration of tocilizumab is a viable option in selected severe and critical COVID-19 patients who do not respond to initial steroids. When given along with steroids, a high suspicion of secondary infections should be kept.
COVID-19; Tocilizumab; Steroids; Acute respiratory failure
Tocilizumab has proven beneficial in severe COVID-19, but its timing, outcome and side effects, when given along with steroids are yet not clear. Our experience shows, administering the drug early to steroid non-responders based on clinical worsening, instead of waiting for the laboratory and radiological worsening can improve the outcomes. A novel coronavirus was first detected in Wuhan, in Hubei Province of China in late 2019 [1]. In February 2020, it was assigned the name COVID-19 by World Health Organization and on March 11th it was declared a pandemic. The coronavirus study group of the International Committee on Taxonomy of Viruses (ICTV) has named it Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2) [2]. Despite all the necessary measures taken to halt this emerging virus, it has spread worldwide [3]. Due to pandemic and associated mortality, astronomical efforts are going on to discover molecules which are effective against SARS-CoV-2. But even after more than one year of pandemic, wide-scale research is still underway to reach a consensus regarding the perfect treatment protocol, which could significantly decrease morbidity and mortality. One of the main reasons behind this may be the unpredictability of the disease progression.
COVID immuno-pathogenesis, has shown the role of heightened cytokine release in causing systemic inflammation and hypoxic respiratory failure [1,4,5]. Hence many drugs altering these mechanisms have been tried as treatment. Among these, to date corticosteroids are the only medications that have been shown to improve survival [6-9]. This may be due to their broad-spectrum anti-inflammatory effects. Another group that is being increasingly recognized, immune modulators like monoclonal antibodies against Interleukin-6 (IL-6) receptors, interleukin-1, CD6 etc. One of the most commonly studied and used drugs among them is tocilizumab which inhibits the binding of IL-6 to both membrane and soluble IL-6 receptors. IL-6 is a pleiotropic cytokine released in response to infection and stimulates inflammatory pathways as a part of the acute phase response. IL-6 inhibitors are currently being used as an anti-inflammatory agent in many conditions like rheumatoid arthritis, cytokine release syndrome due to chimeric antigen receptor T-cell therapy or giant cell arteritis. Infection by the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) induces a dose-dependent production of IL-6 from bronchial epithelial cells [10]. Hence IL-6 inhibitors were tried for its treatment .
Few recent trials have shown good results with the use of tocilizumab in COVID-19. Based on these results, on June 26th, 2021, the United States Food and Drug Administration issued an emergency use authorization for tocilizumab, for the treatment of hospitalized adults band pediatric patients (>2 years) requiring supplemental oxygen. But in such patients, the timing of administration, dosage and effects are still unclear, especially when given in combination with corticosteroids.
To re-evaluate the role of tocilizumab when given along with steroids, we did this retrospective single-centre observational study on all severe and critical COVID-19 patients, who were administered tocilizumab.
Design and setting
We performed a retrospective study at a tertiary care hospital in Northern India after getting approval from the institutional scientific committee.
Hospital electronic medical records were reviewed from April 2020 to February 2021. All consecutive adult (>18 years) patients confirmed as COVID-19 by reverse-transcription polymerase chain reaction assay or rapid antigen test and admitted to the Intensive Care Unit (ICU) with severe and critical disease (Table 1) [11] were noted down. Out of these, those without any evidence of sepsis and treated with tocilizumab were eligible for inclusion. The decision to administer tocilizumab was based on clinical worsening or lack of improvement in oxygen requirement even after>24 hrs of receiving IV methylprednisolone at a dose of 1-2 mg/kg/day, irrespective of the levels of various inflammatory markers. Patients were administered the drug only in the pulmonary or early hyper inflammatory phase (5-20 days from symptom onset) of the disease [12].
| Critical COVID-19 | Severe COVID-19 |
|---|---|
| Defined by the criteria for Acute Respiratory Distress Syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life-sustaining therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy. | · Oxygen saturation<90% on room air. · Respiratory rate>30 breaths/min · Signs of severe respiratory distress (accessory muscle use, inability to complete full sentences) |
Table 1: WHO severity definitions.
Tocilizumab was not given to patients with known hypersensitivity to the drug or any of it's excipients, condition or treatment resulting in ongoing immunosuppression including neutropenia, known or suspected pregnancy, current evidence of any bacterial, fungal or other viral infections, previous history of tuberculosis, and patients with any of following laboratory results out of the ranges, as detailed below at screening.
a) Absolute neutrophil count ≤ 1.0 × 109/L,
b) Platelets<50 × 109/L,
c) Aspartate aminotransferase or Alanine aminotransferase>5 × upper normal limit.
Tocilizumab administration
Tocilizumab was given at a dose of 400 mg intravenously as an infusion over 1 hour, followed by a second dose of up to 400 mg (maximum total dose of 800 mg) within 24 hours if there was no significant clinical improvement.
Standard treatment
Along with this all the patients also received standard treatment which included steroids (1-2 mg/kg of methylprednisolone) for 5-7 days followed by tapering doses, antivirals (remdisivir 200 mg on day 1, followed by 100 mg once daily for 4 days), antibiotics at the discretion of the treating physician, prophylactic dose of low molecular weight heparin and other supportive management.
If not tolerating conventional oxygen support via nasal prongs or face mask, patients were given a trial of High Flow Nasal Cannula (HFNC) and/or Non-Invasive Ventilation (NIV) in ICU to achieve a SpO2 of 88%-93%, if there was no contraindication. Patients were intubated and put on invasive ventilation, based on the following indications:
• Rapid progression of hypoxemia over hours.
• Unable to maintain oxygen saturation (SpO2)>88% on HFNC with a flow of 60 L/minute and FiO2 ≥ 90%.
• Unable to maintain SpO2>88% on NIV with FiO2 ≥ 90% and/ or persistent use of NIV for more ≥ 48 hours.
• Signs of respiratory fatigue (excessive use of accessory muscles of breathing), hypercarbia (pCO2>45 mm Hg), and/or altered mental status).
• Hemodynamic instability.
• Psychomotor agitation, making nursing care impossible and requiring sedation.
Outcome measures
The primary outcomes were the incidence of progression to invasive mechanical ventilation and all-cause mortality at day 28. The secondary outcome measures were clinical improvement assessed using WHO ordinal scale for disease progression (Table 2) [13], change in oxygen requirements and levels of inflammatory markers (C reactive protein (CRP), D-dimer, ferritin and Neutrophil- Lymphocyte Ratio (NLR)) after tocilizumab administration. Life- threatening infections rates and other drug-related side effects were also noted.
| Clinical progression scale | Descriptor | Score |
|---|---|---|
| Uninfected | Uninfected | 0 |
| Ambulatory | Asymptomatic, Infected | 1 |
| Ambulatory | Symptomatic and independent | 2 |
| Ambulatory | Symptomatic and Assistance needed | 3 |
| Hospitalized, mild disease | Hospitalized, No oxygen therapy | 4 |
| Hospitalized, mild disease | Hospitalized, Oxygen by nasal prongs or face mask | 5 |
| Hospitalized, moderate disease | Hospitalized, Oxygen by high flow nasal cannula or NIV | 6 |
| Hospitalized, moderate disease | Intubation and mechanical ventilation, pO2/FIO2 ≥ 150 | 7 |
| Hospitalized, Severe disease | Intubation and mechanical ventilation , pO2/FIO2 ≤ 150 or vasopressor support | 8 |
| Hospitalized, Severe disease | Intubation and mechanical ventilation, pO2/FIO2 ≤ 150 and vasopressors or Dialysis or ECMO | 9 |
| Dead | Death | 10 |
Table 2: WHO Clinical Progression Score (CPS).
Statistical analysis
All statistical analysis was done using SPSS software version 22. The descriptive statistics are reported as mean with standard deviation or median with Inter Quartile Ranges (IQR) for continuous variables and percentages/proportion for discrete variables. Wilcoxon-signed rank test was used to compare the non- normal quantitative values while Fisher’s exact test was applied for the association between categorical variables. Logistic regression was used to find any independent factors associated with poor outcome after tocilizumab administration. All comparative tests were 2 tailed, and a p value of less than 0.05 was considered to be statistically significant.
A total of n=55 patients who did not show clinical improvement even after 24 hours of intravenous steroids were administered tocilizumab. Out of this 4 patients were excluded as they were shifted to another medical facility before day 28 or primary outcome. Hence further n=51 patients were included in the study. The mean age of the patients was 58 (Standard Deviation (SD)-10.9) years, out of which 80% were males and 20% were females. The mean Body Mass Index (BMI) of the patients was 29 kg/m2 (SD- 5.7) with a majority of 41 (80.4%) being overweight. Fever (86.3%) and cough (84.3%) were the most common symptoms, whereas hypertension (62.7%) followed by diabetes (58.8%) were the most common associated co-morbidities. The baseline characteristics of these patients are given in Table 3.
| Age (years) | |
| Mean (SD) | 58(10.9) |
| Sex N (%) | Male-41(80%) |
| Female-10(20%) | |
| BMI (kg/m2) | |
| Mean (SD) | 29(5.7) |
| Symptoms N (%) | Fever- 44(86.3%) |
| Cough-43(84.3%) | |
| Shortness of breath-35(68.6%) | |
| Myalgia-9(17.6%) | |
| Sore throat-5(9.8%) | |
| Diarrhea-4(7.8%) | |
| Vomiting-3(5.9%) | |
| Abdominal pain-2(3.9%) | |
| Oliguria-2(3.9%) | |
| Co-morbidities N (%) | |
| Hypertension | 32(62.7%) |
| Diabetes | 30(58.8%) |
| Coronary artery disease | 11(21.6%) |
| Chronic lung disease | 5(9.8%) |
| Chronic kidney disease | 4(7.8%) |
| Hypothyroid | 2(3.9%) |
| Others | 8(15.7%) |
Table 3: Baseline demographics.
Tocilizumab was administered at a median duration of 8 days since symptom onset and 2 days since hospitalization. At the time of drug administration, 43(84.3%) patients were receiving non- invasive respiratory support (Noninvasive ventilation or high flow nasal cannula), 6(11.7%) patients were receiving conventional oxygen therapy (nasal prongs or venturi mask), and 2(3.9%) were on invasive mechanical ventilation. All of the patients (n=51) were administered tocilizumab based on clinical worsening>24 hours after steroid administration. One or more inflammatory markers were raised in 40/51(78.4%) patients on the day of tocilizumab administration (CRP ≥ 100 mg/L or D-dimer ≥ 1000 ng/ml or IL-6 ≥70 pg/ml (>10 times upper limit of normal) or ferritin ≥ 500 mcg/ml or NLR ≥ 30). Out of the 46 patients in whom HRCT (high-resolution computed tomography) chest was available on the day of tocilizumab administration, 32(69.5%) had CT severity index>15/25 (based on percentage of area involved in each five lobes of lung) [14]. More than 2/3rd (78.4%) of the patients received two doses (800 mg) of tocilizumab (Table 4).
| SFR (SPO2/FiO2 ratio) | |
| Mean (SD) | 164.8(62) |
| FiO2 requirement (%) | |
| Mean (SD) | 61.5(21.4) |
| Number of days since symptom onset (days) | |
| Median (Q1-Q3) | 8(7-11) |
| Number of days since hospitalization days | |
| Median (Q1-Q3) | 2(1-3) |
| Respiratory support at drug administration N (%) | |
| Conventional oxygen support | 6(11.7%) |
| Non-invasive ventilation | 43(84.3%) |
| Invasive mechanical ventilation | 2(3.9%) |
| Increase in one or more inflammatory markers N (%) | 40(78.4%) |
| Radiological severity in HRCT chest (CTSI>15) N (%) | 32 (69.5%) |
Table 4: Other baseline clinical characteristics at tocilizumab administration.
Inflammatory markers
The median baseline values of various inflammatory markers on the day of tocilizumab administration and their trends are given in Table 5. Out of these only CRP showed a significant decrease on day 3 (p<0.001) and day 7 (p<0.001) after tocilizumab administration.
| Median values | P values | ||||
|---|---|---|---|---|---|
| Baseline | Day 3 | Day 7 | Baseline vs. day3 | Baseline vs. day 7 | |
| CRP(mg/L) Median (Q1-Q3) | 114.2(46.3-174.8) | 23.6(10.2-38.4) | 3.6(2.1-5.1) | <0.001 | <0.001 |
| Ddimer (ng/ml) Median(Q1-Q3) | 401(240-574) | 435(254.2-966.7) | 588(267-835) | 0.432 | 0.163 |
| Ferritin (mcg/ml) Median (Q1-Q3) | 541.6(356.6-897.2) | 525.9(252-975.3) | 471(257-595.4) | 0.765 | 0.124 |
| NLR Median(Q1-Q3) | 12.4(7.3-24) | 13.6(7.9-21) | 17.5(9.2-26.8) | 0.807 | 0.358 |
| IL6(pg/ml) Median(Q1-Q3) | 69.8(38.8-153.6) | _ | |||
Table 5: The trend in inflammatory markers (median with interquartile ranges).
A comparison between the levels of various inflammatory markers in those who who had survived versus those who had died on or before day 28 is given in the Table 6. Day 3 ferritin (p=0.026) along with day 7 d-dimer (p=0.041) and NLR (p=0.044) were significantly deranged in those with bad outcome at day 28 (Figure 1).
| Day 28 status | Alive | Died | P-value |
|---|---|---|---|
| Baseline IL6 Median(Q1-Q3) | 53.9(26.5-112.7) | 136.5(51.1-231) | 0.092 |
| CRP (mg/L) Median(Q1-Q3) | |||
| Day 0 | 112.1(43.5-171.5) | 123.7(46.3-200) | 0.314 |
| Day 3 | 22.8(10.2-35) | 38.4(14.4-50.2) | 0.078 |
| Day 7 | 3.6(2.3-4.7) | 3.7(1.7-9.3) | 0.767 |
| D-dimer(ng/ml) median(Q1-Q3) | |||
| Day 0 | 301(222.5-571) | 465(410-577) | 0.07 |
| Day 3 | 359(228.2-902.2) | 659(338.3-3040) | 0.118 |
| Day 7 | 386(252.5-801) | 807(394-3610) | 0.041* |
| Ferritin(mcg/ml) median(Q1-Q3) | |||
| Day 0 | 513.7(315-758.8) | 650(408.3-1036.5) | 0.223 |
| Day 3 | 471.1(234.5-582.2) | 979.8(6646.7-1237.1) | 0.026* |
| Day 7 | 471(234.5-582.2) | 717.1(411-1000) | 0.427 |
| NLR median(Q1-Q3) | |||
| Day 0 | 11.9(7.4-24.4) | 16.9(4.5-20) | 0.635 |
| Day 3 | 12.5(7.7-20.6) | 19.9(10.4-32.2) | 0.104 |
| Day 7 | 11.6(8.2-25.3) | 23.8(14.1-48.7) | 0.044 |
Table 6: Comparative trend in inflammatory markers based on day 28 status.
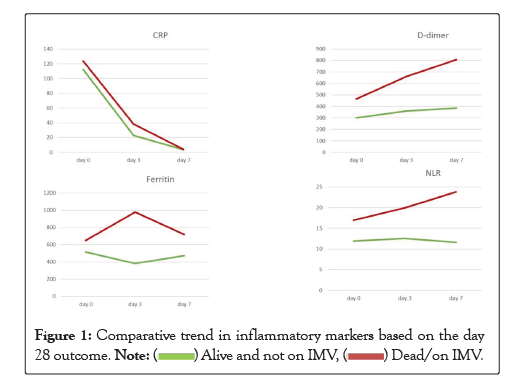
Figure 1: Comparative trend in inflammatory markers based on the day 28 outcome. .
.
Oxygen requirement and clinical progression
Following Tocilizumab administration, there was a significant decrease in oxygen requirement (p<0.001) and clinical progression (p-0.016) (by WHO clinical progression scale) by day 7 (Table 7). The median time to oxygen independence from tocilizumab administration was 7.5 days (IQR, 5-14).
| Day 0 | Day 3 | Day 7 | Day 14 | Day 28 | |
|---|---|---|---|---|---|
| FiO2 (%) | |||||
| Mean(SD) | 61.5(21.4) | 57(24) | 43.6(25.9) | 32.8(22.3) | 25.4(13.2) |
| P values with respect to day 0 | 0.147 | <0.001 | <0.001 | <0.001 | |
| CPS score | |||||
| Mean(SD) | 5.96(0.53) | 6(0.9) | 5.37(1.74) | 4.73(2.76) | 3.88(3.41) |
| P values with respect to day 0 | 0.569 | 0.016 | 0.032 | 0.01 | |
Table 7: O2 requirement and CPS (Clinical Progression Scale) score after tocilizumab administration.
Primary outcome
By day 28, 12/49 (24.5%) patients who were on conventional or non-invasive oxygen support on the day of drug administration progressed to mechanical ventilation. The all-cause mortality at day 28 was 10/51 (19.6%) (Table 8). Out of those died, 9/10 (90%) patients were on invasive mechanical ventilation (Figure 2).
| Primary outcome by day 28 | |
|---|---|
| All-cause mortality N (%) | 10 (19.6%) |
| Progression to mechanical ventilation N (%) | 12(24.5%) # |
Table 8: Primary outcome by day 28. Note: (#) 2 patients who were already on the ventilator while receiving the drug were excluded.
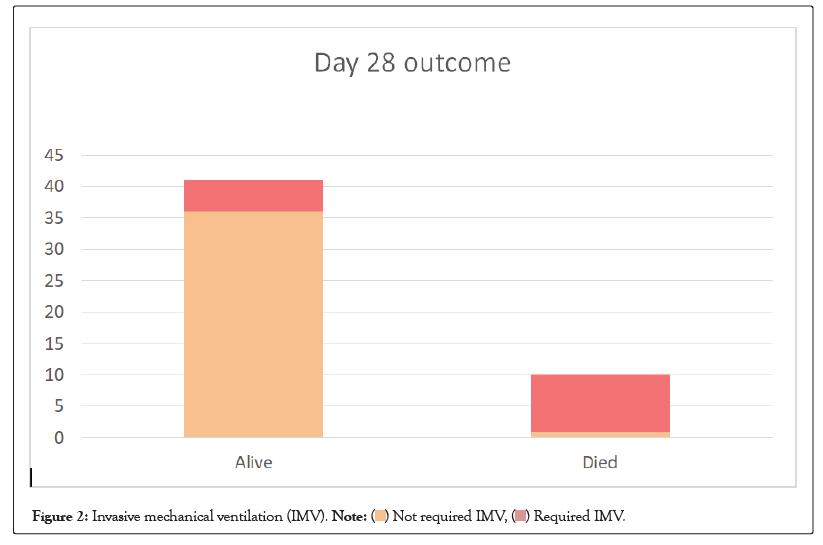
Figure 2: Invasive mechanical ventilation (IMV). .
.
Adverse events
Overall 10/51(19.6%) patients had new onset of serious life- threatening infections which included: bloodstream infections (4), Ventilator-associated pneumonia (4), Hospital-acquired pneumonia (3) and empyema (1). In 18/51 (35.3%) patients, antibiotics were escalated empirically at the discretion of the treating physician. Additionally, 5/51 (9.8%) patients had thrombocytopenia, 3/51 (5.9%) had pneumo-mediastenum or pneumothorax, 1/51 (2%) patient had colonic perforation and 1/51 (2%) patient had transaminitis (enzyme level>5 upper normal limit). There was a significant increase in serious infection rate (70% vs. 7.3%, p<0.001) in the patient who had expired on or before day 28 (Figure 3).
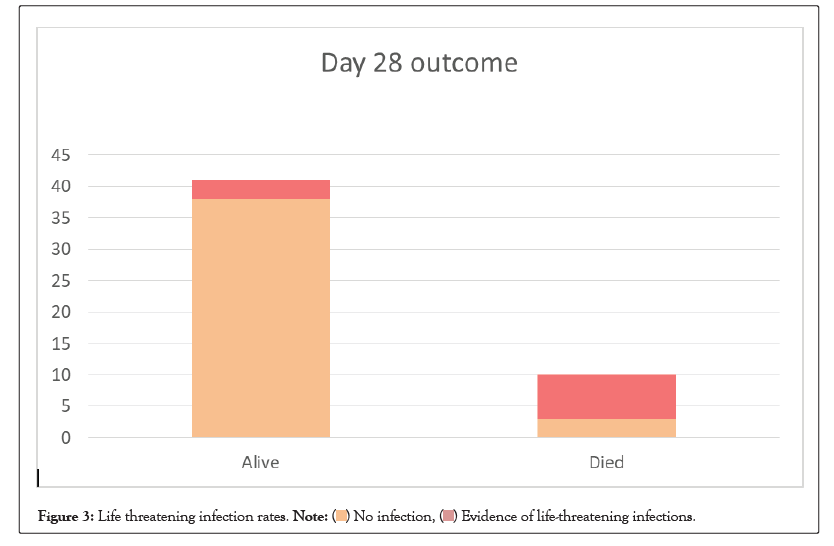
Figure 3: Life threatening infection rates. .
.
Analysis of the above results indicates the usefulness of early and timely administration of tocilizumab in selected severe and critical COVID-19 patients who subsequently worsen or does not respond clinically with initial recommended doses of steroids. This approach can result in a significant decrease in clinical progression and O2 requirement by the end of the first week of treatment.
Most of the initial trials had predominately shown no benefit from IL-6 antagonists in COVID-19 [15,16]. This may be due to the inclusion of less severely ill patients and exclusion of patients receiving any form of respiratory support. Moreover, only a minority of the patients in these trials received tocilizumab in combination with corticosteroids. Some benefits were shown in a few retrospective studies done on the use of tocilizumab in the Indian population with severe and critical COVID-19, still the timing of tocilizumab administration with concurrent steroids, secondary infection rates and long term outcomes were not clear [17,18]. Recently published study by the REMAP-CAP investigators (n=865), included more critical patients, with 70.3% patients on noninvasive ventilation respiratory support and 29% on invasive mechanical ventilation with a median PaO2:FiO2 Ratio (PFR) value of 116.5 [19]. The majority of patients (75.2%) in this study also received glucocorticoids at enrollment or within the following 48 hours. The in-hospital mortality in this study was 27% with the IL6 receptor antagonist group vs. 36% in the control group and they concluded that the treatment with the IL6 receptor antagonists tocilizumab and sarilumab improved outcomes, including survival. The RECOVERY trial is the largest study to date (n-4116) on tocilizumab in COVID-19. This trial also included critical COVID-19 patients (40.9% on non-invasive and 13.6% on invasive respiratory support) and 82% of patients received steroids. This study showed all-cause mortality of 31% in tocilizumab group vs. 35% in the standard care group at day 28 (p=0.002) [20]. Further a recent meta-analysis combining all the RCTs till date on tocilizumab on COVID-19 showed a non-significant decrease in the mortality among critically ill subgroup of patients who are admitted to ICU (all-cause mortality rate was 34.7% vs. 39.6%, p=0.20) [21]. Even though a direct comparison of our data to these previous studies is not possible, the 28-day mortality rate in our patients (19.6%) shows a decreasing trend. This may be because we administered tocilizumab more selectively to ICU patients not responding to steroids rather than giving tocilizumab to all critically ill patients. This approach needs further validation with controlled trials. However, compared to the recovery study, the progression to mechanical ventilation was much larger in our patients (24.5%), this may be because 84.9% of the patients in our group were already on non-invasive respiratory support on the administration of tocilizumab as compared 41% in recovery trial. We believe that our patients were more critical than the patients included in all the above studies because steroid non responders already had a poor clinical outcome. This is also reflected in the mean SpO2/FiO2 (SFR) of 164.2 (SD-62) (corresponding to moderate Acute Respiratory Distress Syndrome (ARDS)) at the time of administration of tocilizumab in our patients. The SFR is a noninvasive surrogate of PFR with good sensitivity and specificity [22]. Moreover 29.4% of our patients were on mechanical ventilation on or before day 28, which predicted high mortality.
We obtained good results, even though tocilizumab in all our patients was administered only based on clinical worsening, irrespective of the levels of their inflammatory markers. This may be due to the following reasons: Firstly, Inflammatory markers like CRP and IL-6 may be very sensitive, but their time to peak serum levels and half-lives are not clear. Moreover, recent studies have shown that systemic levels of cytokines in COVID-19 may not be as high as seen with other causes of sepsis and ARDS [23]. It may be that local inflammation, as evidenced by respiratory dysfunction, is a more useful indicator of which patients will benefit from IL-6 inhibition. Secondly, the CRP levels also decrease significantly after starting steroids [24,25] which is also mirrored in our study, hence its level after initiating steroids may be unpredictable. Thirdly, as the clinical worsening occurs before radiological changes, we did not repeat chest radiology (if the previous imaging was recent (<24 hrs). Finally, the time lag in getting these reports would mean a further delay in the drug administration.
Data from our patients shows that the trend in CRP is a unreliable prognostic marker for disease progression and outcome in those receiving tocilizumab and steroids. However, the trend in other inflammatory markers like Ferritin (at day 3), D-dimer and NLR (at day 7) were significantly deranged in those with poorer outcome. Hence, these paramters can be valuable for prognostication. This needs to be further confirmed in future trials.
As compared to previous studies, the incidence of life-threatening secondary infection (19.6%) was high in our patient cohort. This high infection rate may be attributed to the immuno-supression caused by the combined use of both steroids and tocilizumab. This is a point of concern since 80% of these septic patients had a poor outcome. Hence, a high number of patients (35.3%) needed empirical antibiotic escalation at the discretion of the treating clinician. This may be because the classical clinical manifestations, as well as the diagnostic accuracy of the laboratory parameters of ongoing infections, may be low because of the immunosuppression from steroids and tocilizumab.
Our study has some limitations. Firstly, the descriptive nature and absence of a comparator arm allows us to draw no meaningful conclusions about the population and is the major drawback of the study. Moreover, a comparator arm was difficult in an acute pandemic setting, in view of the unavailability of other treatment regimens for the above clinical scenario. Hence all the indicated patients had received the drug. Secondly, the number of patients observed were very less, thus generalization of the data is not possible. Finally, because of the short follow up period, we were not able to assess the long term safety and adverse effects of tocilizumab.
Our study has some limitations. Firstly, the descriptive nature and absence of a comparator arm allows us to draw no meaningful conclusions about the population and is the major drawback of the study. Moreover, a comparator arm was difficult in an acute pandemic setting, in view of the unavailability of other treatment regimens for the above clinical scenario. Hence all the indicated patients had received the drug. Secondly, the number of patients observed were very less, thus generalization of the data is not possible. Finally, because of the short follow up period, we were not able to assess the long term safety and adverse effects of tocilizumab.
To conclude, selective and timely administration of tocilizumab should be considered in severe and critical COVID-19 patients not responding to steroids. This can result in significant improvement in clinical progression and oxygen requirement. Early and timely administration of tocilizumab is a viable option in selected severe and critical COVID-19 patients who do not respond to initial steroids. When given along with steroids, a high suspicion of secondary infections should be kept.
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
Citation: Assu S, Bhasin D, Sampley S, Singh H, Kaur G, Sekhri K (2022) Survival and Outcomes of Tocilizumab Use in Severe and Critically-ill COVID-19 Patients not Responding to Steroids. J Anesth Clin Res. 13:1057.
Received: 12-Apr-2022, Manuscript No. JACR-22-16963; Editor assigned: 14-Apr-2022, Pre QC No. JACR-22-16963 (PQ); Reviewed: 28-Apr-2022, QC No. JACR-22-16963; Revised: 03-May-2022, Manuscript No. JACR-22-16963 (R); Published: 12-May-2022 , DOI: 10.35248/2155-6148.22.13.1057
Copyright: © 2022 Assu S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.