
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2021)Volume 11, Issue 6
Vancomycin-induced thrombocytopenia is a rare adverse reaction that has been reported in only a select few amounts of medical literature. We describe a case of a critically-ill patient with suspected vancomycin-induced thrombocytopenia who experienced severe thrombocytopenia seven days after discontinuation of vancomycin in the setting of an intracranial hemorrhage. A 48-year old man was admitted to the hospital after complaints of weakness and aphasia, found to have a large basal ganglia hemorrhage. During his hospital course, vancomycin therapy was initiated for suspected infection. On day 7 of empiric treatment, the patient developed thrombocytopenia with a nadir value of 4,000/mm3 with symptoms of active bleeding. It is suspected that vancomycin-induced thrombocytopenia may be derived from an immunological-mediated process. As vancomycin is a commonly-used treatment option for a growing emergence of methicillin-resistant Staphylococcus aureus, clinicians should focus on the work-up of thrombocytopenia in the setting of the intensive care unit and vancomycin usage.
Vancomycin; Thrombocytopenia; Adverse drug reactions; Immunology; Critical care; Toxicology
Thrombocytopenia, which occurs in 14%-44% of adult critically ill patients, is a common hematologic observation in the Intensive Care Unit (ICU) [1,2]. Common causes of thrombocytopenia include but are not limited to sepsis, disseminated intravascular coagulation, consumption processes, and mechanical circulatory support [3]. Medications that are often implicated as the cause of thrombocytopenia include non-steroidal anti-inflammatory drugs, anti-convulsants, heparin, and select antibiotics [4]. Thrombocytopenia that is associated with vancomycin has been reported to occur at a rate of 2% and is mainly associated with prolonged therapy (i.e. greater than 14 days). Vancomycin, a glycopeptide antibiotic, is commonly used in critically ill patients with resistant bacterial infections. We present an uncommon case report of a patient admitted to the ICU suspected to experience vancomycin-induced thrombocytopenia in the setting of a spontaneous intracerebral hemorrhage.
A 48-year old African American male with no significant past medical history presented to the neuroscience intensive care unit at Memorial Hermann-Texas Medical Center after being found down in his home with symptoms of left-sided weakness and aphasia. The initial Computed Tomography (CT) of the brain conducted by the mobile stroke unit revealed a large basal ganglia hemorrhage, complicated by intraventricular extension and mass effect. According to family, the patient was fully functional at baseline with no history of alcohol, tobacco, or illicit-drug use. Additionally, the patient had no medical issues, no known allergies, and no home medications. Upon presentation to the emergency department, the patient had severely elevated systolic blood pressures to 240 mm/ Hg, an initial National Institutes of Health Stroke Scale (NIHSS) score of 17 with a Glasgow Coma Score of 11, and an ICH score of 3, not requiring intubation. Repeat CT imaging revealed a right putaminal hemorrhage measuring 2.8 cm × 5.6 cm with a hematoma volume of 57 ml and a 3 mm midline shift. In the emergency department, the patient was noted to be very restless with persistent elevations in systolic blood pressure requiring nicardipine and esmolol infusions. The patient was eventually intubated due to observations of sonorous respirations.
The patient underwent a right frontal decompressive hemicraniectomy with evacuation of the intracerebral hemorrhage and placement of a left frontal external ventricular drain before transferring to the ICU for post-operative management. Upon admission to the ICU on post-operative day 0, the patient had platelets of 126/mm3 and a mild leukocytosis of 10.1 × 103 with an axillary temperature of 103.6°F that was likely to be reactive from surgery; however, the patient was initiated on cefepime and given one dose of vancomycin (25 mg/kg). The initial procalcitonin level on post-operative day 1 was 0.83 ng/mL, and the chest X-ray revealed diminished lung volumes with bibasilar patchy opacities. From post-operative day 1 to 12, the patient experienced a complex course in the ICU that included multiple investigations of infection, seizures, and coagulopathy work-up. On post-operative day 1, the neurological examination worsened with bilateral upper extremity extension and left eye deviation; a Continuous Electroencephalogram (cEEG) was ordered, fosphenytoin and levetiracetam loading doses were ordered, followed by phenytoin and levetiracetam maintenance doses. On post-operative day 2, phenytoin was discontinued due to an improvement in the patient’s neurological examination and no epileptiform activity on cEEG. Levetiracetam was discontinued on post-operative day 4. The patient underwent a tracheostomy on post-operative day 4 with no complications. On post-operative day 8, the patient was febrile to 103.3°F after completing a 5-day course of empiric cefepime from post-operative day 1 to 5 with no growth shown on respiratory cultures. Blood and urine cultures during this period did not show any growth, however the respiratory culture grew out few yeast and few Haemophilus influenzae. The patient remained febrile with tachycardia, hypertension, and inability to be weaned from the ventilator. Vancomycin was initiated on post-operative day 8 along with re-initiation of cefepime. The respiratory culture on post-operative day 8 revealed beta-hemolytic Streptococcus. On post-operative day 12, the patient was transferred to the stroke unit and antibiotics were de-escalated from vancomycin and cefepime to ceftriaxone. On post-operative day 15, the patient was found to be febrile again to 100.7°F; cefepime and vancomycin were re- initiated at this time. The Computed Tomography Angiography for Pulmonary Embolism (CTA PE) revealed a diagnosis of subsegmental bilateral pulmonary embolisms and the patient was started on a therapeutic heparin infusion.
On hospital day 21 (day 7 of vancomycin for empiric pneumonia treatment), the patient’s platelet count decreased by 48% in 24 hours from 232/mm3 to 121/mm3, see Figure 1 for details.
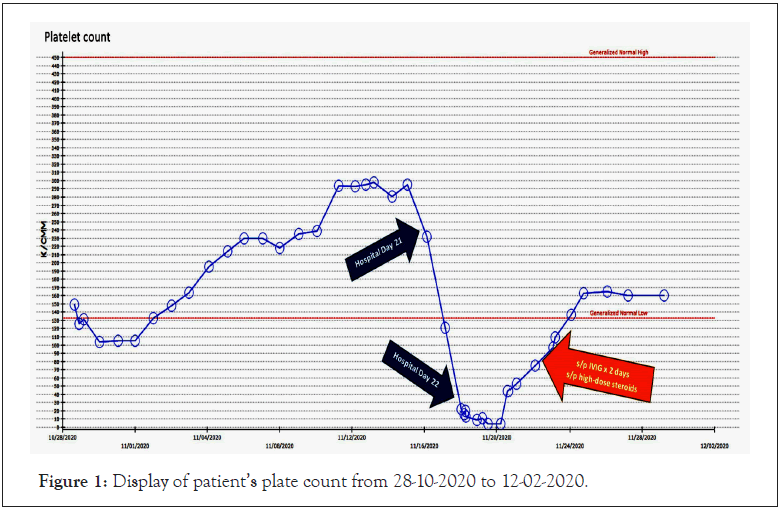
Figure 1: Display of patient’s plate count from 28-10-2020 to 12-02-2020.
On hospital day 22 there was a further reduction by 93% to 9/ mm3. Based on the Naranjo Algorithm for assessing adverse drug reactions, the calculated score was four, suggesting possible adverse reactions from the suspected medication, vancomycin. The patient’s coagulation panel remained stable with PT of 14.8 seconds, INR of 1.15, aPTT of 32.4 seconds, fibrinogen of 576 mg/dl, d-dimer of 3.35 ug/ml and a hemoglobin of 9.5 g/dl. By hospital day 22, the patient had received approximately 20 days of heparin (6 days of therapeutic continuous heparin infusion and 14 days of prophylactic subcutaneous heparin). Heparin was discontinued, two units of platelets were administered, and an Enzyme-Linked Immunoassay (ELISA) test was ordered to rule-out Heparin- Induced Thrombocytopenia (HIT). Additionally, vancomycin was discontinued. On hospital day 23 the ELISA test returned negative for HIT. Hematology was consulted to evaluate the patient in the setting of persistently severe thrombocytopenia, with the differential diagnosis to include Drug-Induced Thrombocytopenia (DITP) versus Idiopathic Thrombocytopenia Purpura (ITP).
Upon admission, the patient’s platelet count was 149/mm3 but recovered to be consistently above 200/mm3 from hospital day 9 to 20. The platelet count declined from hospital day 20 to day 23 to a nadir of 4,000/mm3 with bleeding observed near the site of his percutaneous endoscopic gastrostomy tube. As a result, the patient was aggressively treated for the diagnosis of ITP and received Intravenous Immune Globulin (IVIG) dosed at 1 gm/ kg/day for 2 days in addition to dexamethasone 40 mg every 24 hours for 4 days. Two days after completing IVIG and high-dose steroid therapy, the patient’s platelet count improved to 75,000/ mm3 after 4 days of vancomycin discontinuation. Heparin therapy was re-initiated when the platelet count recovered to 109,000/ mm3 and rheumatology was consulted for work-up of a possible autoimmune process. Initial work-up revealed a positive Anti- Nuclear Antibody (ANA) titer of 1:160, elevated Erythrocyte Sedimentation Rate (ESR), C Reactive Protein (CRP), and positive Lupus Anticoagulant (LA), however, the platelet count was normal prior to hospitalization, thus rheumatology concluded that the patient did not meet clear diagnostic criteria for autoimmune rheumatic disease at this point and suspected thrombocytopenia secondary to medication exposure. Vancomycin-dependent antiplatelet antibodies were not collected; however, the findings from this case suggest that the patient experienced vancomycin- induced immune thrombocytopenia. The patient was transitioned from a continuous heparin infusion to a direct oral anticoagulant and was discharged to inpatient rehabilitation.
The incidence of thrombocytopenia in critically ill medical patients ranges from 35 to 44%, with an even greater incidence witnessed in the critically ill surgical and trauma patient population of up to 65% [5,6]. The primary clinical significance of thrombocytopenia is the associated increased risk of bleeding that further leads to increased mortality in the ICU [5-7]. Sustained thrombocytopenia in the critically ill patient for more than 4 days is associated with a 4- to 6-fold increase in ICU mortality and should be identified for further clinical work-up by clinicians [6,7]. There is an extensive list of differential diagnoses for thrombocytopenia in the ICU, however drug-induced thrombocytopenia is a frequent yet difficult diagnosis due to exposure to multiple agents and the mechanisms of low platelet count associated with other causes in the ICU [2,3].
Vancomycin-induced thrombocytopenia is a rare condition that has only been observed in a few case reports with definite evidence. To date, there is one large case series of 34 patients with clinical suspicion of vancomycin-induced thrombocytopenia, vancomycin- dependent, platelet-reactive antibiotics of immunoglobulin G class with or without immunoglobulin class M [8]. Investigators found that this phenomenon of rapid platelet decline was observed to occur around day 6 of vancomycin therapy, which is the proposed time that is sufficient to mount an immune response. Severe bleeding occurred in 10 patients; however, recovery of platelet counts to baseline in all surviving patients after vancomycin therapy was discontinued. The median time required for the platelet level to return to at least 150,000/mm3 after vancomycin was discontinued was 7.5 days [8]. Ruggero and colleagues describe a patient with a similar clinical course of presumed vancomycin- induced thrombocytopenia after two doses of vancomycin in the setting of no isolated drug-dependent antibody testing [9,10].
The proposed mechanism of thrombocytopenia may involve vancomycin-dependent antibodies that behave similarly to antibodies in patients with quinine-induced thrombocytopenia. The suggested pathogenic mechanism involves the medication binding to the membrane glycoprotein, which induces a conformational change at a remote location for specific binding of the antibody. As a result, this type of drug-dependent antibody does not require the presence of covalent linkage of the drug to the target molecule [8,9]. Features of vancomycin-induced thrombocytopenia that have been reported include extensive ecchymosis and hemorrhage, known as “wet purpura” that is described as a type of bleeding not commonly seen with thrombocytopenia induced by other medications. Thrombocytopenia caused by vancomycin may continue for more than 7 days in the setting of renal failure, as the clearance of the antibiotic may be delayed and serve as a risk factor for worse clinical outcomes [8].
The diagnosis of vancomycin-induced thrombocytopenia in our patient was not confirmed by testing for vancomycin-dependent antibodies in the serum blood samples after exposures to the drug. In a case report investigated by Yamanouchi and colleagues, authors report the clinical utility of flow cytometry test for the differential diagnosis of thrombocytopenia. Flow cytometry utilizes platelet- bound fluorescent signals to measure the presence of anti-platelet IgG antibodies [11]. This approach may be needed to target a specific diagnosis as testing is not widely available. Testing may require a substantial amount of time that may not be clinically feasible to wait for results to arrive prior to discontinuing the suspected offending agent [12]. Testing for drug-dependent antibodies may result in negative findings due to a variety of reasons. This includes lack of sensitivity to detect some antibodies in select assay methods, solubility of medications in water that make it difficult to incorporate in some in vitro assays, and the presence of potential metabolites that is responsible for causing thrombocytopenia rather than the parent drug [12]. However, despite lack of official diagnostic testing, clinical criteria for evaluating thrombocytopenia may be appropriate. In our case, drug administration preceded thrombocytopenia with an observed platelet nadir at 7 days that is consistent with previous cases. Additionally, recovery of platelets to above 100/mm3 occurred on day 6 after discontinuation of vancomycin with other etiologies of thrombocytopenia excluded in the diagnosis.
Treatment of suspected or confirmed vancomycin-induced thrombocytopenia should first consist of discontinuing the suspected or offending agent. Management requires close communication amongst providers to evaluate the causes of thrombocytopenia that may be life threatening requiring aggressive platelet transfusion. Infusion of platelets may be appropriate to stabilize the patient while waiting for further work-up and other therapies to take into effect. Hematology and rheumatology consultation may be warranted if there is high clinical suspicion of immune-thrombocytopenia. In patients with severe thrombocytopenia and symptoms of clinically important bleeding initiation of high-dose corticosteroids with or without intravenous immunoglobulin therapy (IVIG) may be warranted [11]. Therapeutic benefits of IVIG suggest multiple mechanisms that contribute to reducing platelet elimination in immune-related processes [13]. High-dose glucocorticoids also have shown rapid response rates that are were comparable to IVIG, as both treatments are generally well tolerated.
Clinicians should also explore other secondary causes of immune- thrombocytopenia if the primary management of suspected drug- induced thrombocytopenia does not provide adequate response. Autoimmune syndromes, such as antiphospholipid syndrome and systemic lupus erythematosus, may require specific immunoassays for IgG and IgM antibodies. Infectious causes by various viral infections, such as human immunodeficiency virus and hepatitis C virus, may warrant further evaluation based on patient presentation and history. Generally, when the working diagnosis is not responding to adequate therapies, additional monitoring for clinical symptoms and continuation of evaluation to the cause of immune-induced thrombocytopenia is necessary.
This case revealed severe thrombocytopenia, likely in the setting of vancomycin therapy after eliminating all other causes on the differential. Vancomycin-induced thrombocytopenia is a rare condition that can be easily disregarded. Due to the common use of vancomycin and the incidence of thrombocytopenia in the ICU, increased awareness of this condition is needed.
Citation: Chan YH, Allison AT, Escobar AM, Bertani R (2021) Suspected Vancomycin-Induced Thrombocytopenia in a Patient with Intracranial Hemorrhage. J Clin Toxicol. 11:496.
Received: 23-Nov-2021 Accepted: 07-Dec-2021 Published: 14-Dec-2021
Copyright: © 2021 Chan YH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.