Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Case Report - (2024)Volume 13, Issue 5
Introduction and importance: Synchronous occurrence of endometrial and ovarian cancers, particularly when they share the same histological type, presents diagnostic and therapeutic complexities. This case report highlights the challenges and nuances in diagnosing and managing such synchronous malignancies, emphasizing the need for meticulous evaluation and tailored therapeutic strategies.
Case presentation: A 46-year-old female (G.S) presented with chronic pelvic pain and abnormal uterine bleeding. Clinical examination revealed an enlarged uterus and masses in the left iliac fossa and ovaries. Imaging studies, including ultrasound and CT scans, indicated bilateral ovarian masses and peritoneal carcinosis. Histopathological examination following surgical intervention confirmed bilateral infiltrating grade II endometrioid adenocarcinoma involving the endometrium and ovaries.
Clinical Discussion: Synchronous endometrial and ovarian cancers, accounting for a substantial proportion of gynecological synchronous cancers, present predominantly in women around 53 years old, often affecting younger individuals with the endometrioid type. Differential diagnosis necessitates evaluating multiple clinicopathological factors to distinguish between primary tumors and metastatic disease. Recent molecular studies suggest the possibility of a continuum in these malignancies, indicating a potential origin in endometrial foci. Lynch syndrome is prevalent in a subset of cases. Treatment typically involves surgical intervention followed by neoadjuvant radiotherapy and chemotherapy.
Conclusion: The coexistence of synchronous ovarian and endometrial cancers poses diagnostic complexities that demand thorough investigation, including immunohistochemical, molecular, and genomic analysis. Accurate confirmation through targeted sequencing is crucial for tailored management strategies.
Synchronous endometrial and ovarian cancers, Diagnostic challenges, Differential diagnosis, Molecular analysis, Therapeutic strategies, Targeted sequencing
The coexistence of endometrial and ovarian cancers, especially when they share the same histological type, represents a complex diagnostic and therapeutic challenge. Synchronous carcinomas, characterized by the simultaneous occurrence of these malignancies, though not frequent, pose significant clinical intricacies. They are observed in a noteworthy proportion, ranging from 3.1% to 10% of cases among individuals diagnosed with endometrial cancer [1].
This simultaneous manifestation of distinct malignancies in the endometrium and ovary can perplex clinicians due to the intricate differential diagnosis required and the nuanced treatment considerations. When both cancers belong to the same histological type, distinguishing between metastasis from one site to another and the occurrence of independent primaries becomes crucial for appropriate management [2].
In this context, we present a compelling case of independent endometrioid adenocarcinomas affecting both the endometrium and the ovary. This case underscores the significance of meticulous diagnostic evaluation, histopathological examination, and the imperative need for tailored therapeutic strategies in managing such intricate presentations of gynecological malignancies [3].
The rarity of synchronous carcinomas involving the endometrium and ovary emphasizes the importance of comprehensive understanding, accurate diagnosis, and personalized treatment planning. Through this case report, we aim to shed light on the diagnostic intricacies, therapeutic challenges, and unique clinical considerations associated with the co-occurrence of these malignancies [1].
This concerns patient G.S, a 46-year-old individual with O positive blood type, no familial history of neoplasia, and no significant pathological antecedents. In terms of gynecological and obstetric history, she experienced menarche at the age of 12, had regular menstrual cycles, has been married for 11 years, and had two prior miscarriages (2 First-Trimester Spontaneous Abortions). She presented with chronic pelvic pain persisting for 3 months along with abnormal uterine bleeding.
Upon examination, the patient was conscious with well-preserved general condition and normal conjunctivas. She was afebrile with a supple and painless abdomen. Palpation revealed a mass in the left iliac fossa extending to the left flank near the umbilicus. Speculum examination indicated a macroscopically healthy cervix with minimal endometrial bleeding. Vaginal examination revealed an enlarged uterus corresponding to approximately 12 weeks of gestation, a filled Douglas pouch, and a negative β-Human Chorionic Gonadotropin (BHCG) test.
Pelvic ultrasound findings revealed an enlarged uterus measuring 13x7cm with regular contours, a thickened endometrium of 25mm without vascularity on Doppler, and a heterogeneous, poorly defined mass of 11x9cm laterally to the uterus, suggestive of an ovarian mass. Both ovaries were not visualized, and there was no pelvic fluid collection (Figure 1a, 1b).
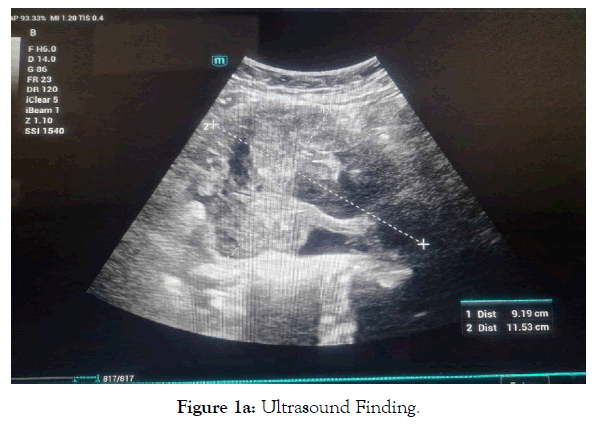
Figure 1a. Ultrasound Finding.
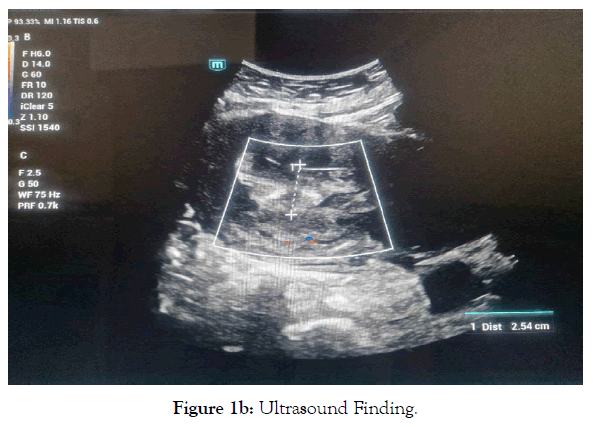
Figure 1b. Ultrasound Finding.
Further evaluation included hysteroscopy with endometrial biopsy revealing complex hyperplasia with complex atypia. The uterus appeared globular with an enlarged endometrium. A left lateral uterine mass of 13x9cm, with a polycyclic contour, tissue-like structure, and enhancement after contrast injection, was observed. Hypodense areas within this mass suggested hypo vascularized zones. Another similar mass measuring 5cm was noted on the right side.
Tumor marker assessment indicated: CA125: 45, ACE: 15.7ng/ml, AFP: 1.03UI/ml, AMH: 0.101ng/ml.
An abdominopelvic CT scan (Figure 2) revealed an enlarged uterus measuring 14cm with a thickened endometrium of 19mm, a heterogeneous cervical mass of 67mm, a left lateral uterine tumor of lobulated solid-cystic contours with irregular tissue-like components of 115mm, to correlate with biopsy results. The left ovary showed a heterogeneous tissue mass measuring 51mm. Bilateral ovarian metastases, peritoneal fat densification with subparietal peritoneal effusion, and peritoneal carcinosis were evident.
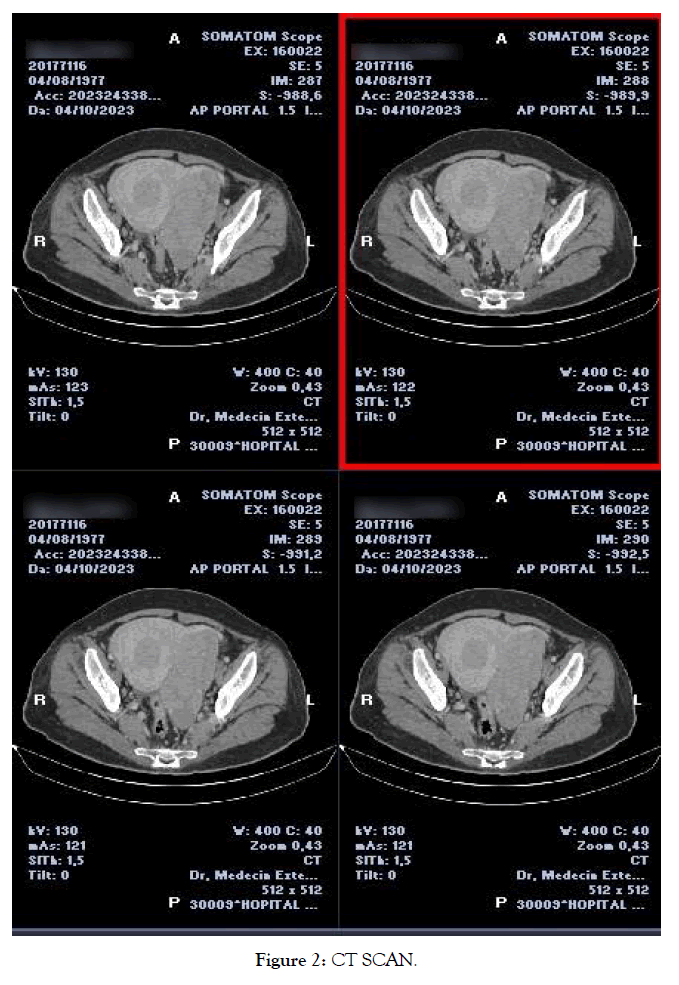
Figure 2. CT SCAN.
Surgical intervention included bilateral annexectomy, which, upon intraoperative frozen section analysis, revealed carcinomatous tissue. Consequently, a total hysterectomy, bilateral lombo-aortic and pelvic lymphadenectomy, omentectomy, and appendectomy were performed.
The final pathological report indicated bilateral infiltrating grade II endometrioid adenocarcinoma involving less than half of the myometrium, thus confirming synchronous ovarian cancers. The cervical tissue showed no tumor invasion, and lymphadenectomy results were negative for cancerous involvement. Moreover, the peritoneum and appendix were free from tumor invasion.
The patient was referred for neoadjuvant chemotherapy and radiotherapy.
The frequency of synchronous ovarian and endometrial cancers accounts for 40 to 53% of gynecological synchronous cancers. The average age of onset for these cancers is 53 years [4], with the endometrioid type tending to appear earlier and affect younger women? Early diagnosis is often made at more favorable stages, resulting in better prognoses [5]. Risk factors associated with the co-occurrence of these two cancers include obesity, perimenopause, polycystic ovary syndrome, an ovulatory cycles, endometriosis, estrogen-producing ovarian tumors, and unopposed estrogen replacement therapy [6].
Abnormal uterine bleeding is the most common presenting complaint. Whenever faced with abnormal uterine bleeding, examination of the adnexa should be combined with endometrial assessment. Distinguishing between primary tumors and metastatic disease requires evaluation of multiple clinicopathological factors. These include the histological similarity of synchronous tumors, the laterality of ovarian tumors, depth of myometrial invasion, presence of vascular invasion, presence of atypical endometrial hyperplasia, and ovarian endometriosis [7]. In 1998, Scully proposed criteria to differentiate between seeding and disseminated cancer, describing three possibilities: endometrial cancer with ovarian metastasis, ovarian cancer with endometrial metastasis, and Synchronous Endometrial and Ovarian cancer [8].
Recent proposals suggest that endometriosis could act as a precursor to gynecological cancers. Some molecular characteristics, such as mutations in ARID1A, PTEN, KRAS, and PIK3CA, microsatellite instability, and loss of p53, are frequent in endometriosis, ovaries, and endometrial cancer [5]. Immunohistochemical examination and molecular analysis are crucial for accurate diagnosis. Recent molecular studies, particularly next-generation sequencing, suggest that this condition should be viewed as a continuum, likely originating in the endometrium or endometrial foci. ER, PR, and bcl2 antibodies seem to play a valuable role, demonstrating different immunolabeling patterns [9].
The most frequent histological type is endometrioid cancer. Between 7% and 9% of women with synchronous ovarian and endometrial cancer suffer from Lynch syndrome [10]. Treatment for early stages involves total hysterectomy, bilateral annexectomy, and bilateral pelvic and lombo-aortic lymphadenectomy. Subsequent treatment includes neoadjuvant radiotherapy and chemotherapy [11].
After treatment, patients with Synchronous Endometrial and Ovarian cancer require gynecological and oncological surveillance every three to four months for the first two years, followed by semi-annual assessments until the end of the fifth year post-treatment [4]. The prognosis for patients with synchronous cancer is better than for those with a single cancer with metastasis [12] evaluated survival rates in patients with stage IA endometrial carcinoma with synchronous stage IA ovarian carcinoma and found that synchronous carcinoma had no significant effect on survival outcomes [13].
This work was reported in line with the SCARE criteria [14].
The association of synchronous ovarian cancer with endometrial cancer poses a diagnostic challenge. It requires a meticulous approach and investigation, including immunohistochemical, molecular, and genomic analysis using targeted sequencing, to confirm it and facilitate appropriate management.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal
None
Not applicable
Not applicable. Our institution requires no ethical approval for case reports.
All authors declare that they have no conflicts of interest.
Not applicable. All authors read and approved the final manuscript.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Citation: Hafsi M, Moussi M, Zouaghi A, Rihani S, Dridi F, Najar S, Maroua S, Mourali M (2024). Synchronous Adenocarcinoma of the Ovary and Endometrium: A Case Report. J Women's Health Care. 13(5):727.
Received: 04-May-2024, Manuscript No. 31185; Editor assigned: 06-May-2024, Pre QC No. 31185; Reviewed: 16-May-2024, QC No. 31185; Revised: 22-May-2024, Manuscript No. 31185; Published: 27-May-2024 , DOI: 10.35248/2167-0420.24.13. 727
Copyright: © 2024 Hafsi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Sources of funding : Not applicable. All authors read and approved the final manuscript.