Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2016) Volume 7, Issue 2
This experiment was conducted to determine possibilities of synergism among insecticidal plants against Z. subfasciatus with a view of augmenting potency and reducing dosage rates. Leaf and seed powders of five insecticidal plants, namely Jatropha curcas (L.), Datura stramonium (L.), Chenopodium ambrosioides (L.), Schinus molle (L.) and Azadrachta indica (A. Juss) were mixed to 1% and 2%w/w unitary and binary formulations. The synthetic insecticide primiphos methyl at the rate of 0.1/100 gm grain dust and untreated grains were used as positive and negative controls, respectively. Most binary formulation had better efficacy than their constituent unitary formulation especially at lower dosage rates. Synergistic combination of botanical powders resulted in highest adult mortality, F1 progeny reduction and lowest weevil perforation index and weight loss comparable to chemical standard primiphos methyl. Among the botanical combinations, bean seeds treated with binary formulation of C. ambrosioides with D. stramonium, J. curcas and S. molle gave the best efficacy in controlling Z. subfasciatus.
<Keywords: Z. subfaciatus; Binary formulation; Botanical insecticides; P. vulgaris; Synergism
The common bean (Phaseolus vulgaris L.) is one of the important food and cash crops in eastern and southern Africa [1,2]. Preharvest and post-harvest damage by insect pests, inter alia, is a major limiting factor of bean production, especially in smallholder farming conditions, under which most beans are grown in the region. Stored beans suffer heavy losses in terms of both quality and quantity mostly by bean bruchids [1]. Common bean weevil, Acanthoscelides obtectus and Mexican bean weevil Zabrotes subfasciatus are the most important species of bruchids attacking stored beans, causing yield losses reaching up to 38% [3,4] Bruchids infestation damage quantity, quality and viability of bean seed [3]. The degree of loss depends on the storage period and storage conditions. Ref. [5] reported an average grain loss of 60% within 3-6 months of storage period due to bean bruchids.
To reduce storage losses due to insect pests, synthetic insecticides have been recommended. However, their use is limited under small scale farming condition due to high costs and infrequent supply [6,7]. Besides, indiscriminate use of insecticides may result in undesirable consequences such as resistance development by the pest, secondary pest outbreaks, wide spread environmental hazards and risk to spray operators [8,9]. For these reasons, development of other alternative control methods such as botanical insecticides have gained significant importance in bruchid management [4,10,11]. Use of botanical insecticides not only confers effective pesticidal effect against bruchids but also serves as ecologically sound and economically feasible control option with low health risks to consumers [8,12]. Different plant extracts may act synergistically to effectively inhibit pest growth and developments compared with a single constituent extract and development of pest resistance is less likely when used over time [13-15].
Even though encouraging efforts that have been made in the last 2-3 decades, to identify botanicals with better insecticidal potential for bruchid management [16-18] limited information is available in their synergistic potential, toxicology, optimal application and species specificity. Moreover, recommended rates were often high which created inconvenience in practical application of botanical. Hence, the current study was undertaken to examine the prospect of synergism among combinations of crude botanical formulations with the objective of enhancing effectiveness of constituent botanical in mixtures and reducing dosage rates. The botanical plants were chosen based on their local availability and their potential for bean bruchids control [17,19]. The insecticidal plants and parts used in this study are shown in Table 1.
| No. | Scientific name | Common name | Parts used |
|---|---|---|---|
| 1 | Azadirachta indica | Neem tree | Seed |
| 2 | Chenopodium ambrosioides | Mexican tea | Leaf |
| 3 | Datura stramonium | Thorn-apple | Leaf |
| 4 | Jatropha carcus | Physic nut | seed |
| 5 | Schinus molle | Pepper tree | Seed |
Table 1: List of botanical plants and parts to be used against Z. subfascitus.
Insect rearing
Adult bean bruchids (Z. subfascitus) were obtained from laboratory culture reared on disinfested common bean variety, Awash-1. The experimental insects were maintained under laboratory condition (27 ± 3°C, 60 ± 10% RH, 12L:12D) at Melkassa Agricultural Research Center (8°24′N; 39°21′E). The food medium (bean seeds) used for insect rearing was first disinfected by keeping the grains in the oven at 40°C for 4 hours and allowed to cool for 2 hrs before use [20]. Infestation was done by introducing 100 parental adults (1:1 sex ratio) in 1 L volume of glass jars containing 250 g of bean grains. The parental adults were sieved off 13 days after oviposition period and the grains were kept under laboratory condition until the emergence of F1 progeny. New generations of adult bean bruchids (Z. subfascitus) obtained from this culture were used in the experiment.
Plant materials and treatment formulations
Fresh plant parts (leaves and seeds) of the botanical plants J. carcus, S. molle and Datura stramonium were collected from MARC and the surroundings. Whereas, plant materials from the other two insecticidal plants C. ambrosioides and A. indica were collected from natural habitat in Addis Ababa (9°1′48″N 38°44′24″E) and Worer Agricultural Research Center (9°20′ 27′′ N and 40°10′ 53′′E), respectively. The plant materials were air dried and crushed separately into fine powder using a pestle and mortar. The resultant powder was further sieved through a 0.25 mm mesh to obtain a fine dust. The powders were weighed into 0.5 and 1 gm samples and then mixed appropriately to constitute binary formulation at either 1 or 2% w/w admixture on 100 gm bean samples. The unitary formulations were weighed into 1 and 2 gm samples and admixed with 100 gm bean samples to represent dosage rates of 1 or 2% w/w respectively.
Toxicity assessment
Healthy disinfected common bean seeds (100 gm) treated with different unitary and binary formulations of botanical insecticide powders were placed in the 1 L volume glass jar. The glass jars tops were covered with nylon mesh to allow aeration and held in place with rubber bands. The effectiveness of the different treatments was assessed by introducing 8 pairs of 2-4 days old bruchids obtained from laboratory culture to the treated and untreated grains. The synthetic insecticide primiphos methyl at the rate of 0.1/100 gm grain dust and untreated grains were used as positive and negative controls, respectively. Percent insect mortality was calculated using Abbott’s formula by counting number of dead insects in each jar 24 hrs, 48 hrs, 72 hrs, 96 hrs and 120 hrs after treatment application/ adult introduction. Adults were considered dead when no response was observed after probing them with forceps. At the end of each assessment, dead insects were removed. The experiment was arranged in completely randomized design (CRD) with three replications.
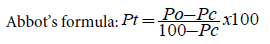
Where Pt=percent (%) mortality; Po=observed mortality; Pc=control mortality
Effect of powders on F1 progeny
After toxicity assessment of plant powders, remaining Z. subfascitus adults on treated and untreated jars were kept for additional 10 days and were sieved and discarded (both live and dead). The infested jars were further maintained under laboratory condition (7 ± 3°C, 60 ± 10% RH, 12L: 12D) until adult emergence and effect of treatments on the F1 progeny were assessed. To avoid overlapping generation, the number of F1 progeny was counted upon emergence for a period of 45 days since the initial date of adult introduction. Percentage reduction in adult emergence or inhibition rate (% IR) was calculated using the following formula:
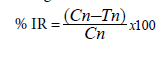
Where Cn=number of newly emerged insects in the untreated (control) jar
Tn=number of insects in the treated jar
Grain damage assessment
To determine grain damage level, samples of 100 grains were taken randomly from the treated and control jars. Both treated and untreated grains were assessed for extent of bruchids damage using exit-holes as a measure of damage to the grain. The number of damaged grains (with characteristic hole) and undamaged grains were counted and weighed. Percentage grains weight loss was calculated using the following formula.
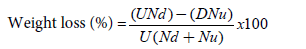
Where U=weight of undamaged grain; Nd=number of damaged grains; D=weight of damaged grain; Nu=number of undamaged grains.
Moreover, grains that are riddled with exit-holes were counted and the percentage damage (PD) and weevil perforation index (WPI) was calculated according to methods in Ref. [21,22] respectively.
PD=(total number of treated grains perforated/total number of grains) × 100
WPI=(% of treated grains perforated/% of control grains perforated+% of treated grains perforated) × 100
Germination test
Germination test was carried out by randomly picking 80 undamaged grains from each treatment jar. Then 20 grains from each treated and control groups were placed separately on a moistened filter paper in Petri dishes and kept at room temperature. Each treatment was replicated four times where healthy grains without botanical insecticide powder application were used as a control. The numbers of germinated grains were recorded starting from the first date of germination. Percent germination was computed using the following formula:
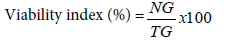
Where NG=number of grains germinated and TG=total number of grains tested in each Petri dish.
Data analysis
All data were checked for normality before they were subjected to analysis. Data which lacked normality were transformed using appropriate transformations method. Data were analyzed with analysis of variance (ANOVA) using General Linear Model (GLM) in SAS software. Significant means were separated using Student-Newma Keuls (SNK) test.
Effects of different botanical powder combinations on bruchids mortality
Results on adult mortality of Z. subfasciatus 24-120 hrs after application of unitary and binary formulations of different botanical powders at 1% w/w and 2% w/w dosage rates on common beans grain are shown in Table 2 and 3 and Figure 1. Significant difference (P<0.001) in adult mortality was observed among different treatments depending on type of botanicals and their combinations, dosage rates and time after treatment application. Significantly higher Z. subfasciatus mortality was recorded under binary botanical formulations compared to unitary formulation at both dosage rates (1% w/w and 2% w/w). For example, mean bruchid mortality recorded 120 hrs after application of unitary formulation was 55% while for binary formulation it was 75%.
| Treatments | Adult mortality (% mean ±SE) | F-value | P-value | ||||
|---|---|---|---|---|---|---|---|
| 24hrs* | 48hrs | 72hrs | 96hrs | 120hrs | |||
| A. indica | 16.67±2.08Ec** | 47.92±4.17Cb | 64.58±5.51Ca | 70.83±4.17Fa | 80.28±2.41Da | 34.73 | P<0.0001 |
| C. ambrosioides | 27.08±4.17Dd | 60.42±2.08Bc | 72.92±2.08Bb | 87.5±3.61Ca | 93.22±3.85Ba | 58.01 | P<0.0001 |
| D. stramonium | 4.17±2.08Gc | 27.08±2.08Eb | 50±3.61Ea | 64.58±4.17Ha | 71.53±7.22Fa | 35.73 | P<0.0001 |
| J. curcas | 22.92±5.51Dc | 47.92±5.51Cb | 68.75±7.2Ca | 79.17±7.51Fa | 87.83±6.47Ca | 14.21 | P =0.0004 |
| S. molle | 14.58±2.08Fc | 35.42±5.51Db | 54.17±4.17Da | 62.5±0.00Ia | 71.81±0.69Fa | 40.13 | P<0.0001 |
| A. indica+C. ambrosioides | 33.33±2.08Cd | 60.42±4.17Bc | 70.83±2.08Cb | 81.25±0.00Ea | 90.97±0.28Ca | 75.44 | P<0.0001 |
| A. indica+D. stramonium | 29.17±4.17Db | 56.25±3.61Ba | 62.5±3.61Ca | 72.92±5.51Fa | 80.42±3.97Da | 17.18 | P=0.0002 |
| A. indica+J. curcas | 31.25±3.61Dc | 54.17±5.51Cb | 70.83±2.08Ca | 81.25±3.61Ea | 88.75±4.39Da | 26.99 | P<0.0001 |
| A. indica+S. molle | 33.33±2.08Cd | 60.42±8.33Bc | 72.92±5.51Bb | 83.33±2.08Da | 88.92±4.58Ca | 16.71 | P=0.0002 |
| C. ambrosioides+D. stramonium | 35.42±4.17Cd | 64.58±2.08Bc | 75.0±3.61Bb | 89.58±2.08Ba | 93.44±2.16Ba | 57.44 | P<0.0001 |
| C. ambrosioides+J. curcas | 22.92±2.08Dd | 47.92±2.08Cc | 75.0±3.61Bb | 87.5±3.61Ca | 94.36±3.61Ba | 82.84 | P<0.0001 |
| C. ambrosioides+S. molle | 35.42±5.51Cd | 58.33±2.08Bc | 70.83±5.51Cb | 85.42±5.51Ca | 90.36±3.61Ba | 21.19 | P<0.0001 |
| D. stramonium+J. curcas | 31.25±3.61Dc | 47.92±5.51Cb | 68.75±6.25Ca | 77.08±2.08Fa | 86.81±1.81Da | 22.52 | P<0.0001 |
| D. stramonium+S .molle | 37.5±3.61Bb | 54.17±2.08Ca | 60.42±5.51Ca | 66.67±5.51Ga | 75.83±6.31Ea | 6.06 | P=0.0097 |
| J. curcas+S. molle | 22.92±4.17Dd | 47.92±4.17Cc | 64.58±5.51Cb | 70.83±8.33Fa | 74.72±3.37Da | 15.45 | P=0.0003 |
| Primiphose methyl | 87.5±7.22Aa | 91.67±4.17Aa | 100±0.0Aa | 100±0.0Aa | 97.92±2.08Aa | 2.12 | P=0.1532 |
| Control (untreated) | 0.00±0.00Ha | 0.00±0.00Fa | 0.00±0.00Fa | 0.00±0.00Ja | 2.08±2.08Ga | 1.00 | P=0.4516 |
| F-value | 23.85 | 20.11 | 20.48 | 25.93 | 28.53 | ||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
*Hours after treatment application; **Means followed by the same letter (s) within a column (upper case letters) and within a row (lowercase letters) are not significantly different using Student-Newman-Keuls (SNK) test (P<0.05). Effectiveness of botanicals and their combinations was determined by computing percent insect mortality (Abbotts 1925) and comparing the mortality data by ANOVA using GLM procedure.
Table 2: Mortality (% mean ± SE) of adult Z. subfasciatus on common bean seeds admixed with unitary and binary formulations (2% w/w) of different botanical insecticide powders.
| Treatments | F1 progeny | % Inhibition Rate (IR) | Weevil Perforation Index(WPI*) | % Weight Loss |
|---|---|---|---|---|
| A. indica | 18.33±0.88c** | 58.89±7.56d | 37.36±3.23b | 1.33±0.45a |
| C. ambrosioides | 1.00±1.0f | 98.33±1.67a | 3.51±3.51e | 0.04±0.04c |
| D. stramonium | 22.33±3.18b | 48.52±13.31e | 32.77±11.56b | 1.00±0.50b |
| J. curcas | 14.00±2.08d | 69.26±5.29c | 18.80±1.02d | 0.95±0.46b |
| S. molle | 10.00±1.73e | 78.33±3.53b | 19.58±2.50c | 0.19±0.18c |
| A. indica+C. ambrosioides | 3.00±1.73f | 92.78±3.89a | 10.50±5.44e | 0.05±0.05c |
| A. indica+D. stramonium | 4.33±2.19e | 91.67±4.19a | 7.00±3.76e | 0.17±0.13c |
| A. indica+J. curcas | 3.33±0.33f | 92.59±1.21a | 10.45±1.38e | 0.18±0.12c |
| A. indica+S. molle | 7.33±0.88e | 83.15±4.38b | 18.90±2.49d | 1.13±0.11b |
| C. ambrosioides+D. stramonium | 1.33±0.88f | 97.59±1.45a | 3.04±1.63e | 0.00±0.00c |
| C. ambrosioides+J. curcas | 1.67±0.33f | 96.48±0.49a | 4.49±1.23e | 0.00±0.00c |
| C. ambrosioides+S. molle | 0.00±0.00f | 100.00±0.00a | 0.00±0.00g | 0.00±0.00c |
| D. stramonium+J. curcas | 7.67±1.20e | 83.15±3.05b | 8.63±1.54e | 0.18±0.09c |
| D. stramonium+S. molle | 8.00±1.15e | 82.59±2.59b | 12.17±1.73e | 0.35±0.10c |
| J. curcas+S. molle | 4.67±1.20e | 90.37±1.03a | 12.02±0.84e | 0.22±0.09c |
| Primiphos methyl | 0.33±0.33f | 99.26±0.74a | 1.19±1.19f | 0.04±0.04c |
| Control (untreated) | 47.00±7.00a | 0.00±0.00f | 50.00±0.00a | 1.5±0.41a |
| F-value | 28.23 | 31.00 | 14.29 | 4.89 |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Table 3: Mean number of F1 progeny produced (mean ± SE), % inhibition rate (IR), weevil perforation index (WPI) and % weight loss caused by Z. subfasciatus on common bean seeds admixed with different unitary and binary botanical powder formulations at 1% w/w dosage rate.
Adult Z. subfaciatus mortality due to botanical insecticide application was directly related to exposure time. Longer duration of exposure after treatment application resulted in significantly higher adult mortality and vice versa for both unitary and binary botanical formulations (P<0.05). For instance, mean adult mortality due to C. ambrosioides+D. stramonium treatment was only 35.42% after 24 hrs while the same botanical formulation caused 89.58% mortality after 96 hrs (4 days) (Table 3). Overall, lowest Z. subfasciatus mortality was recorded 24 hrs after treatment application where as the highest mortality was recorded 96 hrs after treatment application. In the present study, no significant difference in percent adult mortality was observed between 96 hrs and 120 hrs after treatment application except for binary formulation of C. ambrosioides+J. curcas at 1% w/w dosage rate.
Mortality effect of botanicals was dose dependent especially for unitary formulations. An increased Z. subfaciatus mortality was observed at higher doses for unitary formulation. For example, Z. subfasciatus mortality due to J. curcas application at 1%w/w dosage rate was 53.33% which increased to 80.83% at higher dosage rate (2%w/w). On the other hand, notable increase in adult mortality due to higher dose was not observed in most binary formulations. For instance, binary formulations of C. ambrosioides, D. stramonium, J. curcas and S. molle had more or less similar mortality effects at lower and higher application rates. An increase in adult mortality due to higher application rate in binary formulation was observed mainly during J. curcas combination with A. indica and D. stramonium.
Effects of botanical insecticides on weevil perforation index (WPI) and percent grains weight loss
Weevil Perforation Index (WPI) and % weight loss due to Z. subfasciatus on common bean seeds admixed with different application rates of unitary and binary botanical powder formulations is presented in Tables 4 and 5. All botanical insecticide formulations resulted in positive protective effect against damage by Z. subfasciatus, as the WPI values for all treatments were significantly less than 50. Generally, binary formulations showed better protectant ability compared to unitary formulation. For instance, the mean WPI for binary formulation at 1% w/w dosage rate was 8.72% whereas the mean WPI for unitary formulation was 22.41%. WPI was reduced with an increase dosage rate especially for unitary formulation. Binary formulations D. stramonium+C. ambrosioides, C. ambrosioides+J. curcas and S. molle+C. ambrosioides gave the best protection against Z. subfasciatus damage, with WPI value less than 5 at both test doses. This protective effect due to botanical formulation was on par with standard synthetic insecticide, primiphos methyl.
| Treatments | F1 progeny | % Inhibition Rate (IR) | Weevil Perforation Index(WPI*) | % Weight Loss |
|---|---|---|---|---|
| A. indica | 8.67±3.71b** | 82.96±4.86e | 17.35±4.63d | 0.33±0.18b |
| C. ambrosioides | 0.33±0.33b | 99.26±0.74a | 2.42±1.21i | 0.14±0.07b |
| D. stramonium | 11.33±2.33b | 76.11±2.00g | 21.06±1.31c | 0.17±0.05b |
| J. curcas | 6.33±3.33b | 87.78±4.75d | 13.29±4.79f | 0.17±0.17b |
| S. molle | 9.33±1.76b | 78.89±6.19f | 31.41±2.79b | 0.77±0.31b |
| A. indica+C. ambrosioides | 2.33±1.20b | 95.37±2.57b | 4.29±2.97i | 0.18±0.09b |
| A. indica+D. stramonium | 5.33±0.88b | 87.96±2.73d | 19.35±1.95d | 0.46±0.17b |
| A. indica+J. curcas | 2.33±0.88b | 94.26±2.80b | 6.7±2.16h | 0.03±0.03b |
| A. indica+S. molle | 5.67±1.45b | 87.96±2.91d | 13.78±2.31f | 0.17±0.09b |
| C. ambrosioides+D. stramonium | 0.67±0.33b | 98.70±0.67a | 2.14±1.09i | 0.04±0.04b |
| C. ambrosioides+J. curcas | 0.00±0.00b | 100.00±0.00a | 0.00±0.00k | 0.00±0.00b |
| C. ambrosioides+S. molle | 0.00±0.00b | 100.00±0.00a | 0.00±0.00k | 0.00±0.00b |
| D. stramonium+J. curcas | 7.00±1.00b | 84.44±2.94e | 14.94±3.65e | 0.1±0.09b |
| D. stramonium+S. molle | 6.33±0.88b | 86.48±0.19d | 14.07±3.83f | 0.55±0.23b |
| J. curcas+S. molle | 4.33±0.33b | 90.56±0.85c | 11.61±4.91g | 0.22±0.22b |
| Primiphos methyl | 0.33±0.33b | 99.26±0.74a | 1.19±1.19j | 0.04±0.04b |
| Control (untreated) | 47.00±7.00a | 0.00±0.00h | 50.00±0.00a | 1.51 ±0.41a |
| F-value | 22.60 | 70.75 | 21.32 | 4.91 |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
*WPI value above 50 indicate negative protectant ability; **Means followed by the same letter within a column are not significantly different using Student-Newman-Keuls (SNK) test (P<0.05). The data was analyzed by ANOVA using GLM procedure (SAS2002-2008)
Table 4: Mean number of F1 progeny produced (mean ± SE), % inhibition rate (IR), weevil perforation index (WPI) and % weight loss caused by Z. subfasciatus on common bean grain admixed with different unitary and binary botanical powder formulations at 2% w/w dosage rate.
Weight loss due to Z. subfasciatus was significantly reduced (P<0.0001) after application of both unitary and binary botanical formulations compared to untreated control 7 weeks after infestation. The untreated bean grain had the highest weight loss due to damage by Z. subfasciatus. Overall, binary formulations had better effect in reducing weight loss compared to unitary formulation. For instance, mean weight loss after seed treatment with unitary formulation was 41% while that of binary formulation was 15% at 1%w/w dosage rate. Among the botanical treatments, the highest weight loss (65%) was recorded on beans grain treated with A. indica while the lowest weight loss due to S. molle+C. ambrosioides treatment at 1%w/w dosage rate. There was neither seed damage nor weight loss recorded on bean grains treated with binary formulations of C. ambrosioides+J. curcas and S. molle+C. ambrosioides at 2% w/w.
Effects of unitary and binary botanical formulations treatment on percent germination
Germination percent of common bean seeds treated with different unitary and binary botanical powder formulation is presented in Table 5. There was no significant (P>0.05) difference in the percent germination between disinfected common bean seeds treated with different botanical insecticide formulations and untreated control at both dosage rates. The percent germination of bean seed treated with different botanical powder formulations ranged between 92-99%, which was as good as untreated control, indicating botanical treatment didn’t have effect on germination rate.
| Treatments | Percent Germination (mean±SE) | |
|---|---|---|
| 1% w/w | 2% w/w | |
| A. indica | 96.67±1.12a* | 95.00±2.46a |
| C. ambrosioides | 98.75±0.65a | 97.92±0.96a |
| D. stramonium | 97.08±1.14a | 97.50±0.75a |
| J. curcas | 95.42±1.14a | 97.92±0.74a |
| S. molle | 98.75±0.65a | 97.08±1.68a |
| A. indica+C. ambrosioides | 96.67±1.98a | 96.67±1.55a |
| A. indica+D. stramonium | 95.00±1.51a | 96.67±1.67a |
| A. indica+J. curcas | 92.50±1.90a | 97.08±1.79a |
| A. indica+S. molle | 97.92±0.94a | 96.67±1.12a |
| C. ambrosioides+D. stramonium | 92.92±1.99a | 94.58±2.42a |
| C. ambrosioides+J. curcas | 97.50±1.44a | 99.17±0.56a |
| C. ambrosioides+S. molle | 96.25±1.52a | 97.50±1.44a |
| D. stramonium+J. curcas | 94.58±1.68a | 98.33±0.94a |
| D. stramonium+S. molle | 95.42±1.44a | 97.92±1.14a |
| J. curcas+S. molle | 93.33±1.88a | 95.83±1.72a |
| Primiphos methyl | 98.33±0.71a | 98.33±0.71a |
| Control (untreated) | 97.92±0.96a | 97.92±0.96a |
| F-value | 2.03 | 0.69 |
| P-value | P=0.5308 | P=0.7992 |
*Means followed by the same letter within a column are not significantly different using Student-Newman-Keuls (SNK) test (P<0.05). The data was analyzed by ANOVA using GLM procedure (SAS 2002-2008)
Table 5: Effect of unitary and binary botanical formulations treatment on percent germination (mean ± SE) of common bean seeds.
Several studies have been carried on the potential of botanical insecticides in controlling insect pests including stored grain pests; however, only a few studies considered their synergistic combination. Results from the present study demonstrated synergistic potential of different botanical insecticide powders in controlling of bean bruchids (Z. subfasciatus) on stored beans (P. vulgaris). Overall, binary formulations had better effect in reducing damage compared to unitary formulation as assessed by different control parameters such as adult mortality (Table 6), F1 progeny production, percent inhibition, and weevil perforation index (WPI) and weight loss. Moreover, combining more than one botanical insecticide which works synergistically will also make difficult for pests to develop resistance.
| Treatments | Z. subfasciatusmortality (% mean ± SE) | F-value | P-value | ||||
|---|---|---|---|---|---|---|---|
| 24hrs* | 48hrs | 72hrs | 96hrs | 120hrs | |||
| A. indica | 20.83±5.51Ec** | 33.33 ± 9.08Eb | 45.83 ± 9.08Hb | 60.42 ± 2.08Fa | 67.36 ± 5.14Ga | 6.09 | P=0.0095 |
| C. ambrosioides | 27.08±9.08Dc | 41.67 ± 7.51Dc | 50.0 ± 7.22Fb | 70.83 ± 4.16Da | 79.28 ± 2.41Ea | 8.29 | P=0.0032 |
| D. stramonium | 12.5±0.0Fc | 31.25 ± 0.0Eb | 43.75 ± 0.0Ha | 47.92 ± 2.08Ha | 54.58 ± 5.42Ja | 31.57 | P<0.0001 |
| J. curcas | 6.25±3.61Gd | 22.92 ± 4.17Fc | 37.5 ± 3.61Ib | 52.08 ± 4.17Ga | 63.33 ± 1.82Ha | 31.32 | P<0.0001 |
| S. molle | 10.42±2.08Gb | 20.83 ± 5.51Fb | 35.42 ± 2.08Ja | 47.92 ± 4.17Ha | 59.17 ± 2.92Ia | 22.09 | P<0.0001 |
| A. indica+C. ambrosioides | 29.17 ± 7.51Db | 56.25 ± 9.55Ca | 70.83 ± 9.08Ca | 72.92 ± 7.51Da | 79.72 ± 3.37Da | 6.14 | P=0.0092 |
| A. indica+D. stramonium | 12.5 ± 3.61Fc | 39.58 ± 2.08Db | 52.08 ± 4.17Fa | 62.5 ± 3.61Fa | 70.89 ± 2.50Fa | 41.49 | P<0.0001 |
| A. indica+J. curcas | 22.92 ± 2.08Db | 43.75 ± 0.0Da | 47.92 ± 2.08Ga | 66.67 ± 7.51Ea | 75.69 ± 9.98Fa | 9.86 | P=0.0017 |
| A. indica+S. molle | 25 ± 3.61Dd | 43.75 ± 3.61Dc | 60.42 ± 7.51Db | 77.08 ± 9.08Ca | 88.61 ± 7.91Ca | 11.44 | P=0.0009 |
| C. ambrosioides+D. stramonium | 27.08 ± 4.17Dd | 56.25 ± 3.61Cc | 72.92 ± 2.08Cb | 85.42 ± 2.08Ba | 87.22 ± 3.85Ba | 57.63 | P<0.0001 |
| C. ambrosioides+J. curcas | 37.5 ± 3.61Bb | 45.83 ± 4.17Db | 56.25 ± 6.25Eb | 58.33 ± 4.17Fb | 87.08 ± 6.67Ba | 13.45 | P=0.0005 |
| C. ambrosioides+S. molle | 35.42 ± 5.51Cc | 62.5 ± 0.0Bb | 77.08 ± 2.08Ba | 87.5 ± 0.0Ba | 92.22 ± 3.85Ba | 48.07 | P<0.0001 |
| D. stramonium+J. curcas | 20.83 ± 2.08Ee | 39.58 ± 2.08Dd | 50.0 ± 3.61Fc | 58.33 ± 2.08Fb | 61.67 ± 4.35Ga | 30.25 | P<0.0001 |
| D. stramonium+S. molle | 14.58 ± 4.17Fb | 29.17 ± 9.08Fb | 56.25 ± 3.61Ea | 70.83 ± 2.08Da | 74.58 ± 3.63Da | 26.48 | P<0.0001 |
| J. curcas+S. molle | 20.83 ± 4.17Ed | 39.58 ± 7.51Dc | 56.25 ± 6.25Eb | 66.67 ± 7.51Ea | 70 ± 8.32Ea | 8.77 | P=0.0026 |
| Primiphos methyl | 87.5 ± 7.22 Aa | 91.67 ± 4.17Aa | 100 ± 0.0Aa | 100 ± 0.0Aa | 97.92 ± 2.08Aa | 2.12 | P=0.1532 |
| Control (untreated) | 0.0 ± 0.0Ha | 0.0 ± 0.0Ga | 0.0 ± 0.0Ka | 0.0 ± 0.0Ia | 2.08 ± 0.08Ka | 1.00 | P=0.4516 |
| F-value | 16.85 | 13.66 | 17.79 | 22.39 | 18.93 | ||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
* Hours after treatment application; **Means followed by the same letter (s) within a column (upper case letters) and within a row (lower case letters) are not significantly different using student Newman Keuls (SNK) test (P<0.05). Effectiveness of botanicals and their combinations was determined by computing percent insect mortality (Abbotts 1925) and comparing the mortality data by ANOVA using GLM procedure
Table 6: Mortality (% mean ± SE) of adult Z. subfasciatus on common bean seeds admixed with unitary and binary formulations (1%w/w) of different botanical insecticide powders.
Significantly higher adult mortality was recorded in binary formulation compared to unitary formulation. For instance, adult mortality which ranged from 44.58-53.33% due to application unitary formulation of D. stramonium, J. curcas and S. molle increased to over 87% when combined with C. ambrosioides, even at lower dosage rate (1% w/w). This indicated combining different botanical insecticides will enhance their potency in controlling Z. subfasciatus. The current findings concur with previous reports which showed enhanced potency of botanicals in controlling pests when combined as binary formulations. For example, combination of A. indica with a pyrethroid resulted in a more effective management of silver white fly (Bemisia argentifolii), a major greenhouse pest of horticultural flowers [23]. Similarly, biological activities of botanical plants, tobacco (Nicotiana tabacum), Mexican marigold (Tagetes minuta), tephrosia Tephrosia vogelli, and A. indica were significantly enhanced in their binary formulation against common bean weevil, A. obtectus on stored beans reported reduced number of pests on cowpea plants and increased yield of grains as a result of synergistic activity of mixed botanical extract from herbal landraces.
Results from the present study demonstrated reduction in the application rates of constituent individual formulations without compromise in control efficacy due to enhanced potency in combining botanical insecticides. Higher bruchids mortality was achieved after seed treatment with binary formulation of C. ambrosioides with D. stramonium, J. curcas and S. molle at lower dosage rate which was on a par with the mortality recorded due to synthetic insecticide primiphos methyl. Besides, low dose binary formulations were more effective in controlling Z. subfaciatus than most of their constituent unitary botanical formulations at high dosage rate. In the current study, a binary formulation in which C. ambrosioides was included had the best mortality effect, often over 85%. Specifically, combination of C. ambrosioides with D. stramonium, J. curcas and S. molle were most potent as highest Z. subfasciatus mortality was achieved with very small quantity/proportion of botanicals applied. Besides, there was no significant difference in adult mortality due to these binary formulations at lower (1% w/w) and higher (2% w/w) dosage rates. Previous studies have demonstrated reduced application rates of synthetic insecticides due to increased potency of binary formulations.
Toxic effects of unitary and binary botanical formulations in the current study were directly related to exposure time of the pest to the treatments. Highest Z. subfasciatus mortality was recorded at longest exposure periods after botanical treatment and vice versa. It was also found out that mortality effect of botanical insecticides was dose dependent particularly for unitary formulations. An increased Z. subfaciatus mortality was observed at higher doses of unitary formulation. Interestingly, some binary formulations had more or less similar effects at both dosage rate, for example, binary formulation of C. ambrosioides D. stramonium, J. curcas and S. molle. Among individual formulations tested, lowest Z. subfasciatus mortality was recorded by D. stramonium at 1%w/w while the highest mortality was observed by C. ambrosioides at higher rate (2%w/w) application. The current findings are in agreement with the previous report by G/selase and Getu where mortality effect of botanicals was shown to be dose and exposure time dependent.
Overall, bean grain treatment with unitary and binary botanical formulations induced significant high reduction in F1 progeny production by Z. subfasciatus compared to the untreated control. Besides, binary formulations showed better reduction in adult emergence compared to their unitary formulation. Bean grain treatment with binary formulations of C. ambrosioides+A. indica, D. stramonium+C. ambrosioides, C. ambrosioides+J. curcas, S. molle+C. ambrosioides, A. indica+J. curcas resulted in the highest reduction F1 progeny produced. Moreover, there was no significant different in F1 progeny reduction due to synthetic chemical primiphos methyl and the binary formulations. Significantly high reduction in F1 progeny as a result of binary formulations application, demonstrated by none or below unitary adult emergent number, strongly suggested enhanced potency of different botanical combinations. Pest attack is population dependent where high pest populations build up lead to high infestation and damage, which in turn depends on number of emerging adults [24,25]. The highly toxic effects of the binary formulations against F1 progeny in this study indicated the potential of synergists as an effective control option against Z. subfasciatus.
The synergistic effect of botanical insecticides in suppressing in F1 progeny could be due to combined factors such as, increased adult mortality, ovicidal and larvicidal properties of botanical formulations and/or presence of chemicals that interfere with insect feeding [26,27]. Previous investigation on wheat treated with A. indica and A. boonei powder attributed suppression of F1 generation of S. zeamais to high mortality of adult insects which disrupts mating and sexual communication as well as deterring females from laying eggs and affecting developmental stages of insects. Related studies showed botanical powder treatment act as oviposition-deterrent, inhibit oviposition by weakening adult bruchid to lay fewer eggs and kill the hatching larvae afterwards [28,29]. In related study reported neem seed kernel admixed to the groundnuts at the rate of 5% reduced the adult emergence of C. serratus. Even though synergistic combination of botanicals in insect suppression has not been widely examined, several studies revealed the potential of botanical insecticides in reducing F1 progeny production on different insect pests.
Damage by Z. subfasciatus infestation was significantly reduced after treating bean grains with unitary and binary botanical formulations compared to untreated control. Binary formulations had better effect in reducing damage compared to their unitary formulations as judged by low to none weevil perforation index and weight loss. This has further confirmed that combining some botanicals as binary formulation will enhance their biological activity to effectively reduce damage by Z. subfasciatus on stored beans. Among the different botanical synergists, combination C. ambrosioides with D. stramonium, J. curcas and S. molle showed the most effective protecting ability against Z. subfasciatus. This was demonstrated by the least weevil perforation index and percent weight loss recorded after their application at lowest dosage rate (1% w/w). In addition to enhanced efficacy, botanical synergist discussed here have favorable toxicological properties such as rapid degradation, low residues and are safe for the consumer which make them preferred biopesticides in storage pest control.
Results from germination test demonstrated that all botanical powder formulations used to treat bean seeds against Z. subfasciatus didn’t have negative effect in germination percents of the seeds at both dosage rates. Hence, bean seed for planting can be protected and kept viable from storage pest by treating them botanical formulations similar to the grain stored for food purposes. Though this is the first time to test combined effect botanical synergists on germination, previous study on seeds treated with unitary botanical formulation showed no significant effect on the germination rate. Our study results are in agreement with several reports which stated botanical insecticides which provided protection against storage pests didn’t affect seed quality and viability [30-32]. In summary, the findings from the current study underscored synergistic combinations of botanicals enhance effective control of storage pests by optimizing potency of constituent botanicals while reducing dosage rates. Toxicity effect of these binary formulations was comparable with the standard chemical pesticide primiphos methyl. Besides, use of botanical insecticides has several comparative advantages over synthetic insecticides, which include low cost, in the context of small holder farmers, availability, reduced environmental pollution and minimal toxicity to humans and livestock [33]. The insecticidal properties of most of botanical plants studied here have been reported against different insect pests [34,35]. However, none of these studies considered synergistic potential of botanicals and similar insecticidal effects were reported at relatively high dosage rates and after longer exposure time. A new dimension of utilizing synergistic combination of different botanical formulations offers an excellent opportunity to increase the efficacy and reduce application rates of biopesticides in effort to successfully control storage pests of agricultural crops [36]. Hence, the authors recommend incorporation the information/ knowledge generated on synergistic combination of botanicals into regular biorational crop protection practice especially by resource limited small scale bean farmers.
The authors are grateful to Melkassa Research Center of Ethiopian Institute of Agricultural Research for providing necessary laboratory facilities for undertaking research. Technical assistance by Weinshet Belay in insect rearing is highly appreciated. BT received scholarship to undertake this research as part of his MSc study from Ethiopian Ministry of Education and Hawassa University. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.