Journal of Molecular Imaging & Dynamics
Open Access
ISSN: 2155-9937
ISSN: 2155-9937
Research Article - (2013) Volume 3, Issue 1
Synthesis and biological evaluation of the 99mTcN-Gemifloxacin dithiocarbamate (99mTcN-GIND) complex was investigated in terms of radiochemical stability (RCP) in saline, serum, in-vitro binding with Streptococcus pneumoniae (S. pneumoniae) and biodistribution in male Wistar rats artificially infected with living and heat killed S. pneumoniae. The maximum RCP was 98.25 ± 0.30% at 30 min and decreased to 91.25 ± 0.34% within 240 min. The complex showed stable behavior (in-vitro) in serum at 37°C with a 14.35% undesirable side products within 16 h. The complex showed 71.25% in-vitro binding S. pneumoniae. The uptake of the complex in the infected muscle was six times higher than the inflamed and normal muscles of the MWR infected with living S. pneumoniae. The promising (in-vitro and in-vivo) radiochemical and biological behavior posed the 99mTcN-GIND complex as a potential radiotracer for S. pneumoniae infection.
Keywords: Gemifloxacin dithiocarbamate (GIND); 99mTcN-GIND complex; Streptococcus pneumonia; Infection
In the early stages, the identification of infection and its discrimination from inflammation is a critical apprehension of the medical community worldwide. The Nuclear Medicine Imaging (NMI) technology has prevailed over the situation after the failure of the sophisticated techniques such as Computerized Tomography (CT), Magnetic Resonance Imaging (MRI) etc [1,2].
The existing and our recently reported infection imaging agents have shown promising results. The in-vitro and in-vivo results of our recently developed kits encouraged us to seek for more stable and specific infection imaging agents [3-15].
Recently, it has been reported that gemifloxacin (GIN) [7-[(4Z)- 3-(aminomethyl)-4-methoxyimino-pyrrolidin-1-yl]-1-cyclopropyl- 6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid] (Figure 1a) is a new broad spectrum antibiotic effective against Streptococcus pneumoniae (S. pneumoniae), Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis including multi-drug resistant strains, Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, Chlamydia pneumoniae or Klebsiella pneumoniae [16,17].
In continuation to our ongoing study, in the present investigation, the conversion of GIN (Figure 1a) to GIND (Figure 1b) as tetradentate chelator and its radio labeling with technetium-99m through [99mTcN]2+ core has been investigated. The 99mTcN-GIND complex was further evaluated in terms of radiochemical stability in saline, serum, in-vitro binding with S. pneumoniae, percent absorption in the artificially infected MWR with S. pneumoniae.
Materials
Gemifloxacin (GIN) (Shanghai Sciencya Biotechnology Co., Ltd. Shanghai, China), TLC (Merk), succinic dihydrazide (SDH), propylenediamine tetra-acetic acid (PDTA) and all the other chemicals and solvents of analytical grade (Sigma). RP-HPLC (Shimadzu, Japan), well counter, scalar count rate meter (Ludlum, USA), Dose calibrator (Capintech, USA) and Gamma camera GKS-1000 (GEADE Nuclearmedizine system, Germany).
Method
Radiosynthesis of the 99mTcN-Gemifloxacin dithiocarbamate: Gemifloxacin dithiocarbamate (GIND) (Figure 1b) was prepared by using the method described earlier [15]. Thereafter, the 99mTcN-GIND complex (Figure 1a) was synthesized by mixing 0.5 mL (1-2 mCi) of sodium pertechnetate (Na99mTcO4-) with 0.05 mg of stannous chloride dihydrate, 5.0 mg each propylenediamine tetra-acetic acid (PDTA) and succinic dihydrazide (SDH). The reaction mixture is then incubated at room temperature for 10 min. Then 2 mg of GIND (dissolve in normal saline (NS)) was added to the reaction mixture followed by incubation for 10 min at room temperature.
Determination of partition coefficient (P): The 99mTcN-GIND complex, octanol and phosphate buffer (PB) in equivalent quantity was vortexed 5 min at room temperature. The blend was then centrifuged at 5000 g for 10 min. Next, 0.1 mL of the mixture was drawn at different periods and measured for activity in well counter interface with scalar count rate meter (WCSCRM). The following equation was used for the measurement of the partition coefficient (P).
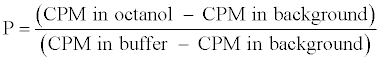
Radiochemical purity (RCP) and characterization: Shimadzu (SCL-10 AVP) HPLC system fitted with (SDP-10 AVP) UV detector operating at 254 nm, (Packard 500 TR series) flow scintillation analyzer, binary pump an online degasser and C-18 (4.6×150 mm) column was used for the radiochemical purity determination and radiocharacterization of the 99mTcN-GIND complex using the reported method [15]. Briefly, 5 µL of the 99mTcN-GIND complex was injected in to the C-18 column of the HPLC system followed by elution of 1 mL/min for 15 min using Water:methanol (W:M) as the mobile phase for 0-3 min (100:00), 3-5 min (60:40), 5-8 min (55:45), 8-10 (25:75), 10-13 (00:100) and 13-15 (50:50). The radiofractions collected during 15 min of elution were measured for activity using WCSCRM.
Radiochemical stability in serum: In serum the stability of the 99mTcN-GIND complex was evaluated using RTLC technique. The 99mTcN-GIND complex (0.2 mL) with 1.8 mL of the serum was incubated at 37°C for 16 h. During the incubation, aliquots at 0, 2, 4, 6, 8, 10, 12, 14 and 16 h were taken and applied to the TLC strips. Next, the strips were developed in saline and CH2Cl2:CH3OH (9:1) (v/v). Thereafter, the developed strips were divided into two equal parts and measured for activity using WCSCRM.
In vitro binding with Streptococcus pneumoniae: In vitro binding of the Streptococcus pneumoniae with 99mTcN-GIND complex was investigated by the reported method [18]. Briefly, to a test tube containing 0.1 mL of the sodium phosphate buffer (Na-PB), 10 MBq of the freshly prepared complex was poured followed by the addition of 0.8 mL (50%, v/v) 0.01 M acetic acid containing approximately 1×108 colony forming units (CFU) of Streptococcus pneumoniae and incubated for 1 h at 4°C with a pH 5. The blend was centrifuged at 2000 rpm for 10 min followed by decanting of the supernatant. Next, the remaining was mixed with 2 mL of Na-BP. The reaction mixture was once again recentrifuged at 2000 rpm for 10 min. The pellets so obtained were measured for in-vitro uptake (%).
Biodistribution in infected MWR: The absorption (%) of the 99mTcN-GIND complex in (per gram) blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle of the MWR infected with living and heat killed Streptococcus pneumoniae (S. pneumoniae) was investigated at 30, 60, 90 and 120 min. Twelve MWR (weight, 150–170 g) were preferred and separated into two groups (A and B) having six MWR in each group. Intramuscularly (I.M.) to the left thigh, 0.2 mL of sterile turpentine oil was injected to each MWR. Next, group A (MWR) were injected (I.M.) with 0.2 mL of living S. pneumoniae (containing around 1×108 CFU) to the right thigh. Likewise, group B (MWR) was injected with 0.2 mL of heat killed S. pneumoniae. After 18 h, intravenously 0.5 mL (18.5 MBq) of the labeled GIND was administered to the MWR of group A and B. Subsequently, the group A and B (MWR) were sacrificed in accordance with the regulations of the Nuclear Medicine Research Laboratory (NMRL), University of Peshawar (Part-I and II). Absorption (percent per gm) in blood, liver, spleen, stomach, intestine, kidney, and infected muscle, inflamed and normal muscle was calculated using WCSCRM.
Radiochemistry and geometry
Gemifloxacin dithiocarbamate (GIND) (Figure 1b) was synthesized from gemifloxacin (GIN) (Figure 1a) using the procedure described earlier [15]. The coordinating groups (sulfur atoms, carboxyl and hydroxyl) of the tetradentate GIND under substitution reaction gave a stable complex of GIND and technetium-99m using the [99mTcN]2+core as shown in Figure 1c. The structure of the 99mTcN-GIND complex was proposed on the likeness with bis (diethyldithiocarbamato) nitride 99mTc complex [19]. The intermolecular complexation could be any permutation of HH-TT, HT-TH etc.
The speculated geometry of the 99mTcN-GIND complex is pyramidal having a TcN:Ligand ratio of 1:1. The 99mTcN-GIND complex showed two radiopeaks at 2.9 and 11.7 min of retention as depicted in HPLC radiochromatogram (Figure 2). The radiopeak at 2.9 min of retention characterized to the [99mTcN]2+ intermediate and the 11.7 correspond to the radiochemical yield of the 99mTcN-GIND complex.
Radiochemically the 99mTcN-GIND complex showed stability in normal saline upto 240 min as shown in Figure 3. The maximum value of the radiochemical stability observed was 98.25 ± 0.30% at 30 min. The value of the radiochemical stability decreased to 91.25 ± 0.34% within 240 min.
Partition coefficient
The P value observed for the 99mTcN-GIND complex was 1.02 ± 0.01 suggesting lipophilicity.
Radiochemical stability in serum
The 99mTcN-GIND complex showed in-vitro stability in serum upto 4 h more than 90% as shown in Figure 4. Thereafter, the growth of undesirable species (de-tagging) lowered the stability value by 16.50% within 16 h.
In vitro binding with Streptococcus pneumoniae
99mTcN-GIND complex showed saturated in-vitro binding with Streptococcus pneumoniae at different intervals as shown in Figure 5. The maximum value of the in-vitro binding was 65.00% and the min was 47.00%
Biodistribution in infected MWR
The absorption (%) of the 99mTcN-GIND complex in (per gram) blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle of the MWR infected with living and heat killed Streptococcus pneumoniae (S. pneumoniae) is given in Table 1. The appearance of the 99mTcN-GIND activity in blood was initially high which was reduced to 4.00 ± 0.18% from 19.50 ± 0.15% within 120 min of the I.V administration. Almost similar behavior was seen in liver, spleen, stomach and intestines of the MWR no matter infected with living or heat killed S. pneumoniae. In kidneys an opposite behavior was seen where the activity of the 99mTcN-GIND complex was low in the beginning of the I.V administration. The activity of the 99mTcN-GIND went up from 8.00 ± 014% to 23.75 ± 0.14% within 120 min. Marginal difference was noted in the uptake of the 99mTcN-GIND complex in kidneys of the MWR infected by living or heat killed S. pneumoniae. The 99mTcN-GIND complex showed higher uptake in the infected muscle than the inflamed and normal muscle of the MWR infected by living S. pneumoniae while no significant difference was observed in the infected, inflamed, and normal muscles of the group B (MWR) infected by heat killed S. pneumoniae.
| Organs /tissues (gm) | Uptakeof the 99mTcN-GIND | |||||||
|---|---|---|---|---|---|---|---|---|
| Group A (living Streptococcus pneumoniae) | Group B (heat killed Streptococcus pneumoniae) | |||||||
| 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | |
| Infected muscle | 6.25 ± 0.18 | 12.00 ± 0.20 | 15.20 ± 0.17 | 12.15 ± 0.16 | 2.50 ± 0.15 | 3.00 ± 0.18 | 3.50 ± 0.16 | 3.00 ± 0.16 |
| Inflamed muscle | 4.50 ± 0.16 | 4.00 ± 0.18 | 3.50 ± 0.14 | 3.00 ± 0.17 | 4.25 ± 0.18 | 4.00 ± 0.14 | 3.50 ± 0.17 | 3.00 ± 0.15 |
| Normal muscle | 2.50 ± 0.14 | 3.00 ± 0.16 | 2.50 ± 0.19 | 2.50 ± 0.20 | 2.50 ± 0.18 | 3.00 ± 0.16 | 2.50 ± 0.17 | 2.50 ± 0.20 |
| Blood | 18.55 ± 0.20 | 10.80 ± 0.16 | 8.00 ± 0.00 | 4.75 ± 0.15 | 19.00 ± 0.17 | 10.65 ± 0.16 | 7.90 ± 0.18 | 4.50 ± 0.20 |
| Liver | 19.00 ± 0.16 | 11.50 ± 0.20 | 9.30 ± 0.18 | 6.00 ± 0.15 | 18.40 ± 0.20 | 11.45 ± 0.15 | 9.10 ± 0.20 | 6.10 ± 0.14 |
| Spleen | 8.70 ± 0.18 | 7.50 ± 0.20 | 6.40 ± 0.14 | 4.20 ± 0.18 | 8.65 ± 0.14 | 7.30 ± 0.17 | 6.25 ± 0.20 | 4.00 ± 0.18 |
| Kidney | 8.00 ± 0.14 | 17.40 ± 0.20 | 20.10 ± 0.16 | 23.75 ± 0.14 | 8.25 ± 0.20 | 19.00 ± 0.14 | 21.30 ± 0.17 | 24.00 ± 0.00 |
| Stomach & intestines | 8.50 ± 0.20 | 7.45 ± 0.16 | 6.75 ± 0.12 | 4.10 ± 0.00 | 8.75 ± 0.14 | 8.00 ± 0.18 | 7.10 ± 0.19 | 4.30 ± 0.18 |
Table 1: Biodistribution of the 99mTcN-GIND complex in artificially infected MWR with Streptococcus pneumoniae.
The appearance of the activity of the 99mTcN-GIND complex in urinary system and disappearance from the circulatory system substantiated the regular path of the excretion of the 99mTcN-GIND complex from the MWR. Figure 6 gives comparative analysis of infected to normal muscle ratios using 99mTc-GIN and 99mTcN-GIND complex at different intervals. Significantly higher uptake ratio was seen in case of 99mTcN-GIND as compared to 99mTc-GIN complex.
The 99mTcN-GIND complex was radiochemically characterized and biologically evaluated in MWR artificially infected with living and heat killed S. pneumoniae. The complex showed radiochemical stability in saline, serum, saturated in-vitro binding with S. pneumoniae and promising biodistribution in MWR with almost six time higher accumulation in the infected muscle as compared to inflamed and normal muscles. Based on the radiochemical stability, in-vitro binding with S. pneumoniae and six time higher absorption in the infected muscle of the MWR, validated the feasibility of the 99mTcN-GIND complex as prospective infection imaging agent.