Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2024)Volume 14, Issue 4
In this study, Poly (Polyethylene Glycol Anhydride) (PPEGA) copolymers synthesised with Maleic Anhydride (MA) and Poly(Ethylene Glycol) (PEG) using two different catalysor; para toluene sulfonic acid and 1,8-Diazabicyclo [5.4.0] undec-7-ene as polymeric SSPCM. PPEGA block copolymers were Solid-Solid Phase Change Materials (SSPCMs) as new Phase Change Material (PCM). The PEG bound to the side chains of the copolymers stored latent heat as it passed from crystal phase to the amorphous phase. Characterization and thermal specifications of SSPCMs were determined by Fourier Transform Infrared (FT-IR) and Differential Scanning Calorimetry (DSC). DSC results showed a SSPCM transition at 36°C-54°C temperature range and 163 Jg-1-153 Jg-1 enthalpy. All findings indicated that the prepared PPEGA copolymer good Thermal Energy Storage (TES).
Maleic anhydride; PEG; Solid-solid phase change; PCM; Latent heat
Presence of different monomers in a polymer molecule can lead to production of materials of commercial importance. PCMs are capable of storing latent heat energy during phase change and then releasing the stored energy to the environment. PCMs and mixtures have different uses textiles, electronics, biomedical and biology and so on. PCMs can be examined in 4 sections as liquid-gas, solid-gas, solid-liquid and solid-solid. Liquid-gas and solid-gas phase transitions are not preferred because they cause significant volume changes. Rather, solid-liquid and solid-solid phase changes are preferred because of very small volume changes [1].
PEG is a non-toxic material that has excellent thermal storage properties and can maintain chemical stability during long-term use and PEG has high fission energy. SSPCMs can store highly latent heat at the time of conversion from crystal phase to amorphous phase. SSPCMs do not produce toxic substances, pollute the environment and do not need a different energy source as they perform latent heat storage through phase transition.
Therefore it is an environmentally friendly application and so on MA is used in small applications such asartificial sweeteners, food enhancers, paper processing, water treatment chemicals [2].
SSPCM investigations with high TES characteristics include several PEG-based polymeric SSPCMs such as polyurethanegraft- PEG, cellulose diacetate-graft-PEG, polypropylene-graft- PEG, cellulose-graft-PEG, poly(vinyl alcohol)-graft-PEG and Poly(styrene-co-maleic anhydride)-graft-PEG as SSPCMs have been prepared or modified by various methods, PPEGA was synthesized new SSPCM. PPEGA stores latent heat through the change phase of PEG bound. Characterization of SSPCMs was revealed by FT-IR and latent heat storage capability was measured and interpreted by DSC. Therefore, MA-PEG (PPEGA) bloc copolymer can be used as energy storage material in food packaging materials [3].
In the pursuit of sustainable and advanced materials for various industrial applications, the development of innovative polymeric materials has become a focal point.
Among these, Poly (Polyethylene Glycol Anhydride) (PPEGA) copolymers have garnered significant attention due to their promising properties such as biodegradability, biocompatibility, and tunable thermal characteristics. These copolymers, particularly when synthesized and characterized with precision, hold great potential for application in food packaging as polymeric Phase Change Materials (PCMs). The utility of PCMs in food packaging lies in their ability to maintain thermal conditions, thus preserving the quality and extending the shelf-life of perishable goods. This study focuses on the synthesis and characterization of PPEGA copolymers using different catalysts to optimize their performance for such applications [4].
The significance of PPEGA copolymers in food packaging is underscored by their unique ability to undergo phase transitions at specific temperatures, which can be strategically utilized to absorb, store, and release thermal energy. This property is particularly valuable in maintaining the integrity of temperaturesensitive food products during transportation and storage. The synthesis process of PPEGA copolymers involves the copolymerization of Polyethylene Glycol (PEG) and anhydride monomers. The choice of catalyst in this process plays a crucial role in determining the efficiency and characteristics of the resulting copolymers. Different catalysts can influence the molecular weight distribution, thermal properties, and overall performance of the PPEGA copolymers, making the selection of an appropriate catalyst a critical aspect of the synthesis process [5]. Maleic anhydride, PEG Mw: 4000 g mol-1, Para-Toluenesulfonic acid and 1,8-Diazabicyclo [5.4.0] undec-7- ene catalysors purchased from Aldrich Company.
Synthesis of PPEGA copolymers
MA-PEG copolymers have been synthesized ring opening reaction and PEG to MA copolymer as shown in scheme (Figure 1). The reaction was established in a nitrogen atmosphere containing a 500 mL dispenser apparatus with reflux condencer, mechanical stirrer. MA and PEG at calculated amounts 20:19 molar ratio. The reaction system was catalyzed by p-toluene sulfonic acid at 160°C for 2 hours. Furthermore, the same procedure was performed for the other MA-PEG copolymer of the 1,8-Diazabicyclo [5.4.0] undec-7-ene catalyst at a 20:19 ratio [6].
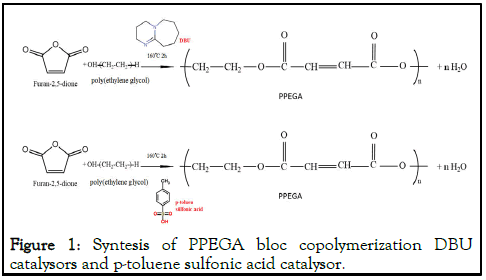
Figure 1: Syntesis of PPEGA bloc copolymerization DBU catalysors and p-toluene sulfonic acid catalysor.
Characterization
FT-IR spectra of SSPCMs were performed with FT-IR spectrophotometer (JASCO 430 model) at 4000 cm-1-400 cm-1 wave range and KBr disk. Thermal energy storage characteristics were determined by Perkin Elmer Jade model DSC in the range of -15°C to 90°C and at a speed of 10°C min-1.
FT-IR spectroscopy analysis of MA-PEG copolymers
Ester and carboxylic acid group of MA-PEG (Mw: 4000) produced tensile vibration at 1710 cm-1. It can be considered that decrease in O-H functional group with change in peak at approximately 3500 cm-1. Maleic anhydride has characteristic peaks 2922/2851 cm-1, 1464 cm-1, 1067 cm-1 and 721 cm-1. MAPEG (Mw: 4000) polymer reduces O-H tensile vibration of MA in range of about 2500 cm-1-3000 cm-1 and decrease in characteristic peak of 1066 cm-1. MA copolymerization FT-IR spectrums shown in Figure 2.
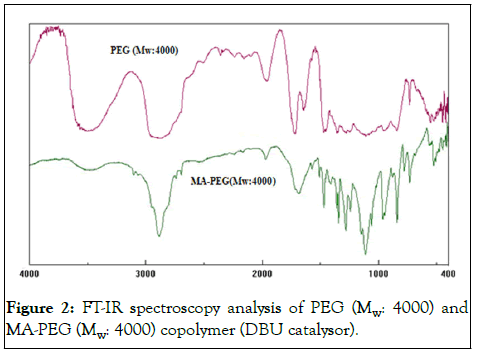
Figure 2: FT-IR spectroscopy analysis of PEG (Mw: 4000) and MA-PEG (Mw: 4000) copolymer (DBU catalysor).
Thermal reliability of MA-PEG copolymers
When the DSC curve of MA-PEG (Mw: 4000) copolymer was examined, melting and solidification temperature difference was measured as 18°C and melting and solidification enthalpy difference as 8 Jg-1 (Figure 3), DSC curve of the MA-PEG 4000 copolymer synthesized with DBU catalyst, melting and solidification temperature difference is 14.8°C and enthalpy difference of melting and solidification is 10.2 Jg-1. It is close to the phase change temperature of PEG 4000 with a melting point of 55.6°C [7]. However, the melting enthalpy decreased by about 84 Jg-1 (Figure 4). DSC analysis results of the produced copolymers are shown in Figure 3,4 and Table 1 respectively.
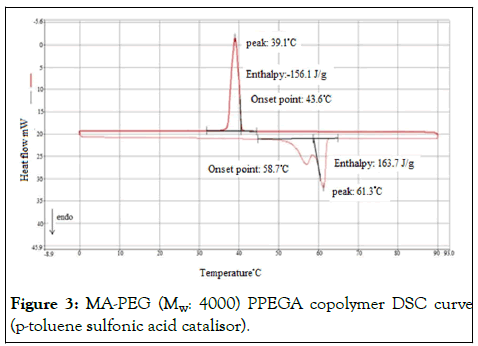
Figure 3: MA-PEG (Mw: 4000) PPEGA copolymer DSC curve (p-toluene sulfonic acid catalisor).
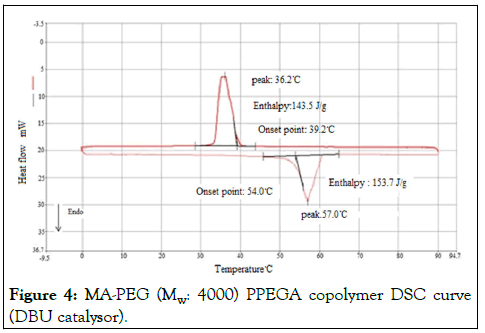
Figure 4: MA-PEG (Mw: 4000) PPEGA copolymer DSC curve (DBU catalysor).
| Heating period | Cooling period | |||
|---|---|---|---|---|
| Ts-s (°C) | ∆Hs-s, (J g-1) | Ts-s, (°C) | ∆Hs-s, (J g-1) | |
| PEG 4000 | 40.5 | 171.5 | 53.9 | 174.2 |
| MA-PEG (Mw: 4000) (DBU) | 54 | 153.7 | 39.2 | -143.5 |
| MA-PEG (Mw: 4000) (Pt.s.a) | 58.7 | 163.7 | 43.6 | -156.1 |
Note: MA: Maleic Anhydride; PEG: Polyethylene Glycol; DBU: 1,8-Diazabicyclo[5.4.0]undec-7-ene; Ts-s: Solid-solid heating and cooling temperature; Pt.s.a: p-toluen sulfonic acid
Table 1: The latent heat thermal energy storage specifications of pure PEG and synthesized SSPCMs.
The synthesis of PPEGA copolymers typically involves the Ring- Opening Polymerization (ROP) of anhydrides in the presence of PEG [8]. This method allows for the incorporation of PEG into the polymer backbone, imparting flexibility and thermal responsiveness to the material. The choice of catalyst is pivotal in this process, as it influences the reaction kinetics, molecular weight distribution, and structural properties of the copolymers. Common catalysts used in the synthesis of PPEGA copolymers include stannous octoate (Sn(Oct)2), organocatalysts such as 1,5,7-Triazabicyclo[4.4.0]Dec-5-ene (TBD), and various metal complexes.
Stannous octoate, a widely used catalyst in ROP, offers high efficiency and control over the polymerization process. Its usage results in copolymers with desirable molecular weights and low polydispersity indices, which are essential for consistent thermal properties. On the other hand, organocatalysts like TBD provide a metal-free alternative, which is particularly advantageous for applications in food packaging where the absence of metal residues is critical. TBD facilitates the polymerization process by acting as a strong base and nucleophile, promoting high conversion rates and yielding copolymers with high thermal stability and controlled degradation rates [9,10].
The characterization of PPEGA copolymers involves a series of analytical techniques to assess their molecular structure, thermal properties, and phase change behavior. Gel Permeation Chromatography (GPC) is commonly employed to determine the molecular weight distribution and polydispersity index of the copolymers. Differential Scanning Calorimetry (DSC) is used to evaluate the thermal properties, including the melting and crystallization temperatures, which are critical for understanding the phase change behavior of the material [11].
Fourier Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR) spectroscopy provide insights into the chemical structure and composition of the copolymers. These techniques can confirm the successful incorporation of PEG and anhydride units into the polymer backbone. Additionally, Thermogravimetric Analysis (TGA) is utilized to study the thermal stability and degradation behavior of the copolymers, which is essential for determining their suitability for food packaging applications [12].
The application of PPEGA copolymers as PCMs in food packaging leverages their phase change properties to maintain desired temperature conditions. During the phase transition, the copolymers absorb or release thermal energy, which can help in mitigating temperature fluctuations that could otherwise compromise the quality and safety of food products. The effectiveness of PPEGA copolymers in this application depends on their thermal properties, such as melting point, latent heat of fusion, and thermal conductivity [13].
By selecting appropriate catalysts during the synthesis process, the thermal properties of PPEGA copolymers can be finely tuned to match the specific requirements of food packaging. For instance, copolymers with lower melting points can be used for packaging refrigerated food items, while those with higher melting points are suitable for products that require higher storage temperatures. The biodegradability of PPEGA copolymers also adds an environmental benefit, as they can reduce the accumulation of plastic waste associated with traditional food packaging materials.
PPEGA block copolymer were synthesized for food packaging material. The copolymers gained latent heat storage feature from the crystalline phase to amorphous Phase of Polyethylene Glycol (PEG) bound to skeleton. Structures of copolymers were determined by FT-IR and similar study of poly (ethylene graft maleic anhydride), it was observed that tensile vibrations of 2923/2850 cm-1 decreased. MA-PEG (Mw: 4000) polymer reduces the O-H tensile vibration of MA in the range of about 2500 cm-1-3000 cm-1 and decrease in the characteristic peak of 1066 cm-1 MA copolymerization. Ester and carboxylic acid group of MA-PEG produced tensile vibration at 1710 cm-1. DSC curve of MA-PEG 4000 copolymer was examined, melting and solidification temperature difference was measured as 18°C and melting and solidification enthalpy difference was measured as 8 Jg-1, DSC curve of the MA/PEG 4000 copolymer synthesized with DBU catalyst, melting and solidification temperature difference is 14.8°C and enthalpy difference of melting and solidification is 10.2 Jg-1. It is close to the phase change temperature of PEG 4000 with a melting point of 55.6°C. However, the melting enthalpy decreased by about 84 Jg-1, melting and solidification temperature difference is 13.0°C and the enthalpy difference of melting and solidification is 16.1 Jg-1 with the effect of the catalyst, energy storage capacity increased and the melting point decreased. DSC measurements due to PEG chains attached to the main chain show that the copolymers have phase transition temperatures of 40°C-58°C and have high latent heat storage capacity in the range of 153 Jg-1-183 Jg-1.
Citation: Ertugral TG (2024) Synthesis and Characterization of Poly (Polyethylene glycol Anhydride) (PPEGA) Copolymers with Use of Different Catalysts for Application to Food Packaging as Polymeric Phase Change Materials (PCMs). J Phys Chem Biophys. 14:391.
Received: 07-Jan-2020, Manuscript No. JPCB-24-3071; Editor assigned: 10-Jan-2020, Pre QC No. JPCB-24-3071 (PQ); Reviewed: 24-Jan-2020, QC No. JPCB-24-3071; Revised: 22-May-2024, Manuscript No. JPCB-24-3071 (R); Published: 19-Jun-2024 , DOI: 10.35841/2161-0398.24.14.391
Copyright: © 2024 Ertugral TG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.