Journal of Theoretical & Computational Science
Open Access
ISSN: 2376-130X
ISSN: 2376-130X
Research Article - (2017) Volume 4, Issue 1
This paper is concerned with the synthesis and hyphenated DFT-experimental characterization of dioxovanadium(V) complexes of semicarbazone ONO-donor ligands, LH [where, LH = N-(4’-benzoylidene-3’- methyl-1’-phenyl-2’-pyrazolin-5’-one)-semicarbazone (bmphp-semH), N-(4’-butylidene-3’-methyl-1’-phenyl-2’- pyrazolin-5’-one)-semicarbazone (bumphp-semH), N-(4’-iso-butylidene-3’-methyl-1’-phenyl-2’-pyrazolin-5’-one)- semicarbazone (iso-bumphp-semH) or N-(3’-methyl-1’-phenyl-4’-propionylidene-2’-pyrazolin-5’-one)-semicarbazone (mphpp-semH)] and were prepared from ethanol-methanol mixed solvent (1/10) solutions of bis(acetylacetonato) oxovanadium(IV) complexes of the above ligands by oxidizing with atmospheric oxygen (bubbling air) for 2-3 days. The composition and formulae of complexes were confirmed by various physiochemical analysis, viz., percentage of different elements, magnetic susceptibility, conductance, FT-IR, UV-Visible and mass spectrometry. One of the representative complexes, cis-[VO2(bmphp-sem)(H2O)] was investigated at the convergence of DFT and experimental formulation interface. The standard B3LYP/LANL2DZ combinations were used to arrive at the approx of geometry optimization, charge distribution and molecular orbital descriptions. The global reactivity parameters like absolute electronegativity (χabs) and absolute hardness () have also been involved. From the overall studies it has been found that the compounds possess cis-octahedral structure.
<Keywords: DFT, Experimental, Dioxovanadium (V), Acylpyrazolone, Electrostatics
Acylpyrazolone are attention-grabbing class of pyrazole scaffolds fused to a chelating arm [1] and their synthetic strategies have been evaluated since 1959 [2]. Pyrazolone derivative have been extensively investigated on account of their wide range of pharmacological activities [3]. The search for pyrazolones with excellent therapeutic action [4] and their incorporation into the pharmacopoeia as an antipyretic and, later on, as an analgesic and anti-inflammatory agent is quite fascinating. Nevertheless, the time to time human trials [5] and accompanied toxicity hindrances, added eager to their research scenario and search for less toxic new anti-inflammatory drugs [6] or the preparation of new compounds with antifungal [7], antitumor [8] and antihyperglycemic [9] activities remained an active goal. Very recently, the application of such compounds in apoptosis of Hela cancer cells [10] has been reported. Their metal complexes have shown peculiar redox behavior [11]. The structural determinations of such complexes have gained much attention at both experimental as well as theoretical approaches [12,13].
Semicarbazones on the other hand are also molecules of great interest due to their potential pharmacological properties [14-17]. A variety of semicarbazones and their metal complexes have been reported to possess properties of wide variation in their stereochemistry and modes of bonding through oxygen and azomethine nitrogen atoms [14]. The metal binding properties in semicarbazones are tailored and appended by tagging with different additional donor atoms involving substituted aldehydes or ketones [18-21] witnessed by a number of reports of the same field of investigation [22-24]. Vanadium(V) complexes have been shown to ensue stereo-chemically flexible coordination geometries [25]. The redox behaviour, including V(V)/V (IV) or V(IV)/V (III), increases the versatility of this element in the biological milieu [26]. Unraveling the role of vanadium in normal mammalian metabolism [27] and enlightening its potential therapeutic application have attained mammoth interests [28-30].
Density-functional theory (DFT) is a powerful method for predicting the geometry of vanadium compounds [31-36] including large molecules [37]. The application of theoretical force constants to attain Cartesian representation is useful for the assumption of molecular symmetry. Generally molecular charge topography and orbital analysis provide ample information regarding various reaction parameters [38].
Vanadium in the oxidation state V has been found more potent cell inhibitor and hence such flexibility of this metal represents the importance in regulatory effects of osteoblasts and is a proof to devise more efficient catalytic. To extend seek for more such types of efficacious compounds of vanadium with neutral charge [28], a systematic study of synthesis and combined DFT-experimental characterization of VO2+ complexes of acylsemicarbazones is hereby reported.
Materials and methods
3-Methyl-1-phenyl-2-pyrazolin-5-one (Lancaster, UK), benzoyl chloride and propionyl chloride (Thomas Baker Chemicals Ltd., Mumbai), butyryl chloride (S. D. Fine, Chemicals, Baroda), iso-butyryl chloride (E. Merck, Germany) and semicarbazide hydrochloride (Sisco Chem. Industries, Mumbai), vanadium pentaoxide [E. Merck (India) Ltd., Mumbai], sodium acetate (Fluka AG Co., Switzerland) were used as received. All chemicals used were of analytical reagent (A. R.) grade.
Preparation of different 4-acyl-3-methyl-1-phenyl-2-pyrazolin- 5-one (amphp) derivatives
The amphp derivatives were prepared by a slight modification of Jensen’s method [39]. Into a one liter 3-necked quick fit flask containing DMF (100 mL) and carrying a dropping funnel, a mechanical stirrer and a reflux condenser was placed 3-methyl-1-phenyl-2-pyrazolin-5- one (0.098 M, 17 g). A solution was obtained by gentle heating and stirring. Calcium hydroxide (0.140 M, 10 g) was added and benzoyl chloride (12 mL)/butyryl chloride (10.33 mL)/iso-butyryl chloride (11 mL)/propionyl chloride (8.98 mL) was added drop wise within 3-5 min. The reaction was exothermic and the reaction mixture became a paste. The mixture was allowed to cool and then refluxed with stirring for 1 h on a sand bath during which period the bright yellow complex formed initially turn yellowish brown. The decomposition of complex was carried out by pouring the reaction mixture into a 3N half liter chilled dilute hydrochloric acid solution. As a result yellowish brown solid was obtained that was filtered using sintered glass crucible. Distilled water was used as washing agent and washing was continued until colourless washings were obtained. The final product was dried in air and recrystallized from n-heptane.
Preparation of semicarbazones
Ethanolic solution of semicarbazide hydrochloride (0.05 M, 7.35 g) was first neutralized with sodium acetate solution (0.05 M, 13.9 g). Into this 25 ml of ethanolic solution of bmphpH (0.05 M, 12.20 g), bumphpH (0.05 M, 12.20 g), iso-bumphpH (0.05 M, 12.20 g) or mphppH (0.05 M, 11.5 g) was added and was allowed to reflux for 3 h at 60°C with constant stirring. A greenish yellow coloured precipitate was formed that was followed by filtration and washing several times with ethanol. The product was dried in a desiccator over anhydrous calcium chloride (Scheme 1).
Preparation of dioxovanadium (V) complexes
The parent compound, bis(acetylacetonato)oxovanadium(IV), [VO(acac)2], was prepared by the method of Patel [40]. A hot methanolic solution (25 mL) of [VO(acac)2] (0.001 M, 0.265 g) was added to the methanolic solution of 25 mL of the respective Schiff base, bmphp-semH (0.001 M, 0.335 g), bumphp-semH (0.001 M, 0.301 g), iso-bumphp-semH (0.001 M, 0.301 g) or mphpp-semH (0.001 M, 0.287 g) and was refluxed for 4 h. It was cooled and filtered. The filtrate was collected and the precipitate was dissolved in ethanol-methanol (1:10) and again filtered. The filtrate thus obtained is again added to the above filtrate, which is then kept for air oxidation at room temperature for 3-4 days, with occasional shaking the resulting compound is collected and recrystallized from methanol.
Elemental analysis
Carbon, Hydrogen and Nitrogen were determined micro-analytically at CDRI, Lucknow. The percentage of vanadium in each of the complexes was determined volumetrically [41] using 0.02 M KMnO4 solution as an oxidizing agent in the presence of sulfurous acid.
Physical methods
Solid-state infrared spectra were reordered in KBr pellets using Perkin-Elmer model 1620 FT-IR spectrophotometer. 1HNMR spectra in DMSO-d6, elemental analyses (micro-analytically) and mass obtained spectra were recorded at Sophisticated Analytical Instrumentation Facility (SAIF), CDRI, Lucknow. Thermo gravimetric analysis of the complexes was performed on a Perkin-Elmer Thermo analyzer at SAIF, IIT, Bombay. Electronic Spectra were recorded using UV-VIS-Near IR Spectrophotometer (Carry-5000), Agilent Technology, Germany. Magnetic measurements were performed by Gouy`s method using mercury (II) tetrathiocyanatocobaltate (II) as calibrant.
DFT calculation
All the calculation presented in this paper was performed by DFT methods using Gaussian 09 program package [42]. The hybrid exchange-correlation Becke3–Lee–Yang–Parr (B3LYP) functional and LANL2DZ basis sets were used [43,44]. Gauss View 5.0 package was used to have visual display of different harmonic oscillations and to obtain various graphic views of molecular charges and shapes of distinctive molecular orbitals. All the parameters were entertained from the optimized structure (gaseous phase) of the representative compound conditioned to the absence of negative frequency.
Synthesis and characterization
Synthesis: The dioxovanadium (V) complexes were prepared according to Scheme 2.
Where LH=bmphp-semH, bumphp-semH, iso-bumphp-semH or mphpp-semH.
The synthesized air stable complexes were found thermally stable and the respective decomposition temperatures along with some physical parameters are given in Table 1. From their solubility test it was found that the complexes are insoluble in most of the organic solvents and have shown maximum solubility in DMF. The formulation of these complexes are based on their elemental analysis, FT-IR, 1H-NMR, mass and electronic spectral studies, Thermo gravimetric, magnetic and conductance determinations.
| Compounds Empirical formula (M.W.) |
Found (calcd.), (%) | Color | Decomp. Temp. (°C) |
ΛM (Ω-1cm2mole-1) |
Yield (%) |
|||
|---|---|---|---|---|---|---|---|---|
| C | H | N | V | |||||
| bmphp-semH C18H17N5O2 (335) |
64.56 (64.47) |
5.12 (5.07) |
20.76 (20.89) |
- | Yellow | 200 | - | 72 |
| bumphp-semH C15H19N5O2 (301) |
59.36 (59.80) |
6.25 (6.31) |
23.45 (23.25) |
- | Yellow | 160 | - | 66 |
| iso-bumphp-semH C15H19N5O2 (301) |
59.75 (59.80) |
6.53 (6.31) |
23.43 (23.25) |
- | Yellow | 163 | - | 69 |
| mphpp-semH C14H17N5O2 (287) |
58.64 (58.53) |
5.84 (5.92) |
24.53 (24.39) |
- | Yellow | 169 | - | 62 |
| [VO2(bmphp-sem)H2O](1) C18H18N5O5V (435.76) |
49.60 (49.32) |
4.16 (4.35) |
16.07 (16.25) |
11.68 (11.35) |
Pepper-ment | 258 | 12.6 | 60 |
| [VO2(bumphp-sem)H2O](2) C15H20N5O5V (401.3) |
44.89 (44.36) |
5.02 (5.28) |
17.46 (17.58) |
12.69 (12.85) |
Lime | 250 | 14.5 | 72 |
| [VO2(iso-bumphp-sem)H2O] (3) C15H20N5O5V (401.3) | 44.89 (44.56) |
5.02 (5.65) |
17.46 (17.32) |
12.69 (12.52) |
Light olive | 235 | 16.3 | 68 |
| [VO2(mphpp-sem)H2O](4) C14H18N5O5V (387.27) |
43.42 (43.25) |
4.68 (4.25) |
18.09 (18.36) |
13.15 (13.52) |
Cascade Green | 263 | 14.6 | 65 |
Table 1: Analytical data and some physical properties of the synthesized Schiff-bases and its complexes.
Infrared spectral studies: The important infrared spectral bands of the acylsemicabazone ligands and complexes along with their tentative assignment are given in Table 2. The acylsemicarbazones used in the present investigation may exist in three keto-enol forms (Figure 1). The combined experimental and theoretical IR spectra of a representative complex are given in Figures 2 and 3. All the four acylsemicarbazones exhibit a weak band centered at 3360-3410 cm-1 for ν(OH), a strong band at 1681-1695 cm-1 for ν(C=O) (semicarbazide skeleton) and another strong band due to ν(C=N) (azomethine) at 1612-1650 cm-1. The presence of NH vibrational mode of hydrazide at 3125-3138 cm-1 and stretching frequency of -NH2 at 3340-3380 cm-1 and 3214-3238 cm-1 was observed in the infrared spectra of all the acylsemicabazones. Hence culminates that the semicarbazone ligands under the question exist in enol form [II] in the solid state. Recently for such chemical moieties the hyderogen bonding between C=O oxygen and adjacent NH- hydrogen stems the same assumption [45].
| Compounds | νs(VO2) | νas(VO2) | ν(C=O) Semi | ν(C=N) Azomethine |
ν(C=N) Pyrazolinring |
ν(C-O) Enolic |
ν(NH) | ν(NH2) | ν(OH) |
|---|---|---|---|---|---|---|---|---|---|
| (bmphp-semH) | - | - | 1695 | 1650 | Merged with ν(C=N) azo | 1210 | 3138 | 3348 3216 |
3360 |
| (bumphp-semH) | - | - | 1694 | 1612 | Merged with ν(C=O) | 1250 | 3130 | 3340 3214 |
3390 |
| (iso-bumphp-semH) | - | - | 1695 | 1615 | Merged with ν(C=N) azo | 1210 | 3138 | 3380 3230 |
3410 |
| (mphpp-semH) | - | - | 1681 | 1620 | Merged with ν(C=N) azo | 1256 | 3125 | 3350 3238 |
3380 |
| [VO2(bmphp-sem)H2O](1) | 860 | 952 | 1659 | 1595 | Merged with ν(C=N) azo | 1261 | 3148 | Merged with ν(OH) | 3420 |
| [VO2(bumphp-sem)H2O](2) | 814 | 909 | 1609 | Merged with ν(C=O) | Merged with ν(C=O) | 1270 | 3140 | Merged with ν(OH) | 3440 |
| [VO2(iso-bumphp-sem)H2O](3) | 817 | 908 | 1615 | 1593 | Merged with ν(C=N) azo | 1280 | 3130 | Merged with ν(OH) | 3380 |
| [VO2(mphpp-sem)H2O](4) | 839 | 979 | 1634 | 1590 | Merged with ν(C=N) azo | 1278 | 3110 | Merged with ν(OH) | 3471 |
Table 2: IR spectral data of the synthesized Schiff-bases and their complexes.
In the light of enol form [II], these four semicarbazone ligands possess seven potential donor sites: (i) the ring nitrogen N1 (ii) the ring nitrogen N2 (iii) the cyclic enolic oxygen (OH), (iv) azomethine nitrogen, (v) the hydrazide nitrogen, (vi) amide oxygen and (vii) amide nitrogen. The coordination of ring nitrogen N1 is unlikely due to presence of bulky phenyl group on it [46]. The appearance of ν(C=N2) (cyclic) mode 1593 cm-1 at almost the same position {here merged with ν(C=N) (azomethine)} compared to ν(C=N2) (cyclic) at ~1590 cm-1 of the uncoordinated ligand, after complexation gestures that the ring nitrogen N2 in these ligands is found to be inert towards coordination to vanadium. The coordination binding of enolic oxygen after deprotonation hints the disappearance of ν(OH) at 3360-3410 cm-1 in the spectra of the complexes. But due to ν(OH) of coordinated water (vide infra), it creates ambiguity to clarify the coordination of enolic oxygen. However, metal-enolic oxygen bond is confirmed by ν(C-O) (enolic) [47] at 1261-1280 cm-1 compared to ν(C-O) (enolic) at 1210-1256 cm-1 in the uncoordinated ligands.
The ν(CO) mode of amide carbonyl group observed at 1681-1695 cm-1 in the ligands is shifted to lower wave numbers [48] and appears at 1609-1659 cm-1 in these complexes. This suggests the bonding of the amide carbonyl oxygen to vanadium in all these complexes. The acylsemicabazones in the present discussion display a sharp and strong band due to ν(C=N) of the azomethine group at 1612-1650 cm-1. The observed low energy shift [49] of the band in the complexes at 1590-1595 cm-1 suggests the coordination of the azomethine nitrogen to vanadium. The vanadium binding with azomethine nitrogen, cyclic enolic oxygen and amide carbonyl oxygen as discussed above favours the formation of the complexes having six and five membered rings in coordination sphere [50]. In view of this, the coordination of hydrazide nitrogen and amide amino nitrogen is ruled out. This is further supported by the no appreciable change in the ν(NH) (hydrazide nitrogen) and ν(NH2) (amide amino group) of the ligands after complexation. The overall infrared spectral studies conclude that the acylsemicarbazones used in the present study behave as monobasic tridentate ONO-donor ligands.
All the metal chelates also show medium/broad band at 3327-3471 cm-1 due to ν(OH) mode because of the presence of lattice/ coordinated water in them. Besides, two sharp bands at 908-979, and 814-860 cm-1 (experimental), 1045 and 1027 cm-1 (theoretical) correspond to νas(O=V=O) and νs(O=V=O) modes, respectively of the cis-VO2 moiety [51-53] are also discussable. Figure 3 indicates the similarities between theoretical and experimental FT-IR results and hence ascertains the true value of proposed functional group vibrations. Hence, the involved level of theory of computation is quite reliable. The FTIR spectra of two representative Schiff bases are given in Figures 2 and S1. While, Figures S2-S4 are infrared spectra of the synthesized complexes.
Electronic spectral studies: The electronic spectroscopic insights of two representative compounds were gained from their 10-3 M DMF solution and the results are given in Table 3. These two complexes displayed two intra-ligand transitions in the ultraviolet region. The 3rd spectral peak at ~378 nm in the complexes is due to ligands → metal charge transfer (LMCT) transition. These results are comparable to the data reported elsewhere [54,55] for dioxovanadium(V) complexes. The theoretical electronic spectrum of representative complex was calculated using the TD-DFT method with the PCM solvent model, using DCM as the solvent. The core information regarding oscillatory strengths, transition energies and excitation coefficients were obtained for complex (1). Transitions bearing largest oscillator strengths are retained, however for a long wave part the transitions with small oscillator strengths are also given in Figures 4a and 4b and the respective theoretical electronic spectrum data have been shown in Table 4.
| Complexes | λmax (nm) | ν (cm-1) | Peak Assignment |
|---|---|---|---|
| [VO2(bmphp-sem)(H2O)] | 245 | 40816 | Intra ligand Transitions |
| 260 | 38441 | Intra ligand Transitions | |
| 378 | 26455 | Ligand to Metal Charge Transfer Transitions (L® M) |
Table 3: Electronic spectral data of the synthesized complexes.
| ΔE (eV) | λ/nm Calc. | f | λ/nm Exp. | Assignments |
|---|---|---|---|---|
| 3.0308 | 409.07 | 0.0219 | 401 | Ligand to Metal Charge Transfer Transitions (L® M) |
| 3.2811 | 377.87 | 0.0146 | - | π- π* intra ligand charge transfer |
| 3.3615 | 368.84 | 0.0083 | - | π- π* intra ligand charge transfer |
| 3.4786 | 356.42 | 0.0368 | - | π- π* intra ligand charge transfer |
| 3.6004 | 344.36 | 0.1427 | 347 | π- π* intra ligand charge transfer |
| 3.7256 | 332.79 | 0.0079 | 300 | π- π* intra ligand charge transfer |
Table 4: Theoretical electronic spectrum of the complex [VO2(bmphp-sem)(H2O)], from Ref. [1] calculated with the TD-DFT method in DMSO solvent.
Conductance measurements: From molar conductance determination the respective assignments of the synthesized complexes in 10-3 M DMF solution have been found in the range of 12.6-16.3 ohm-1 cm2 mol-1 (Table 1). From the results the non-electrolytic nature of these complexes is inferred. The strong donor capacity of DMF, the displacement of anionic ligand and change of electrolyte type are supposed the reasons that develop the non-zero reading [56].
Magnetic measurements: As expected for dioxovanadium (V) compounds [40], the observed magnetic moments (0.23-0.78 BM) show diamagnetic behaviour of this class of complexes.
Mass spectrometry insights: The fast atomic bombardment (FAB) type of mass spectrometry carried out for a representative complex cis-[VO2(mphpp-sem)(H2O)] (4), Figure 5 was recorded on a JEOL SX 12/DA-6000 Mass Spectrometer/Data system using Xenon/Argon (6 KV, 10 mA) as the FAB gas in the m/z range 86.35-1111.42. The mass spectral peaks observed at 136, 137, 154, 289 and 307 m/z are matrix [m-nitrobenzyl alcohol (NBA)] peaks. The appearance of a peak at 365 m/z is consistent with molecular mass of the present compound excluding one water molecule present therein, which is supposed to be lost before ionization. The other mass spectral signals observed at, 365, 441, 524 and 525 m/z in the complex might be because of the following types of ion aggregations:
[VO2(mphpp-sem)]+ (369)-4[H]+=365
[(mphpp-sem)]+ (285)+[matrix]+ (154)+2[H]+=441
[VO2(mphpp-sem)]+ (369)+[matrix]+ (154)+[H]+=524
[VO2(mphpp-sem)]+ (369)+[matrix]+ (154)+2[H]+=525
The mass spectral data shows close agreement with the supposed molecular structure of the complex (4).
1NMR spectral studies: The proton NMR spectrum of a representative compound, namely, cis-[VO2(bmphp-sem)(H2O)] (1) Figure 6, was recorded in DMSO-d6, using TMS as a reference. This compound displays a multiplet of proton signal due to aromatic protons of phenyl group at δ 7.22-7.94 ppm and a singlet at δ 10.11 ppm due to –NH proton of hydrazide moiety. The proton signals at δ 3.33 and 1.34 ppm are most probably due to proton groups such C-NH2 (b), -C-CH3 (a), respectively present in this compound. The proton signal at 2.51 ppm is most probably due to solvent (DMSO-d6) taken. The nonappearance of enolic proton signal at ~ 12 ppm in the complex (deprotonation of -OH) points the coordination of enolic oxygen with the metallic centre. The indexing of various proton groups is given in the Figure 5. The 50V NMR spectrum of the model compound, cis- [VO2(bmphp-sem)(H2O)] (1), Figure 7 was recorded in DMSO-d6. The complex exhibits a strong resonance at ca. -508.90, as expected for the dioxovanadium(V) complexes having a mixed O/N donor set.
Thermogravimetric studies: Thermo gravimetric curve of cis- [VO2(bmpph-sem)(H2O)] (1), was recorded in the temperature range of 25-1000°C with the heating rate of 15°C /min (Figure 8). The compound (1) shows a weight loss of 4.44% at 170°C (Calcd. weight loss of one mole H2O, 4.13%) corresponding to the elimination of one molecule of coordinated water [52]. This compound shows further weight losses beyond 300°C and upto 980°C in three steps, which unfortunately could not be correlated separately. However, the final weight loss of 75.6% observed at 980°C corresponds to the elimination of one (bmphp-sem) ligand group and one water molecule (calcd. weight loss, 81%). The final residue (obs.=24.4%) roughly corresponds to V2O5 (calcd.=19.0%).
The proposed sequential representation of the thermal decomposition of complex may be shown as below:
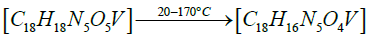
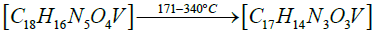
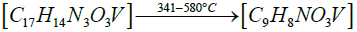
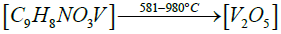
DFT studies
Molecular geometry: The main elements of optimized geometry of the title compound, cis- [VO2(bmphp-sem)(H2O)] including interatomic distances and the angle of orientation of different bindings are shown in Table S1. The structure appears to have a little distorted octahedral geometry (Figure 9). It is observed that, the length of V=O bond fall in the range of 1.61-1.62 Å for dioxovanadim complex it is very close agreement with experimental values [49]. The computed bond lengths, such as, V-O(2),(oxo) V-O(3),(oxo) V-O(4), (enolic), V-N(5)(azo), V-O(6)(ketonic) and V-O(7) water in the present complex are 1.619, 1.612, 1.888, 2.531, 2.021, and 2.403 Å, respectively. The bond angles in the coordination sphere of the complex, such as, O(3)-V(1)-O(2)(105.117), O(4)-V(1)-O(2)(103.987), O(4)-V(1)-O(3) (105.388), N(5)-V(1)-O(2)(87.204), N(5)-V(1)-(3)(166.601), N(5)- V(1)-O(4)(73.222°), O(6)-V(1)-O(2)(96.288), O(6)-V(1)-O(3) (102.729), O(6)-V(1)-O(4)(138.928), O(6)-V(1)-N(5)(72.574), O(7)- V(1)-O(2)(163.250), O(7)-V(1)-O(3) (89.962), O(7)-V(1)-O(4) (76.915), O(7)-V(1)-N(5)(76.915) and O(7)-V(1)-O(6)(73.902) (°) revealed the octahedral geometry of the compounds [56].
Molecular orbital approach: Highest Occupied Molecular Orbitals (HOMO) and Lowest Unoccupied Molecular Orbitals are very significant elements of theoretical molecular design [57]. The intermolecular and intramolecular interaction can be predicted by this way. Hence, the name “Frontier orbitals” emerged. The HOMO is electron donor and LUMO the electron acceptor sites, and the HOMO-LUMO gap speculates the molecular hardness and softness of a compound [58]. Six molecular orbitals (MOs), viz., [HOMO- 2], [HOMO-1], [HOMO], [LUMO], [LUMO+1] and [LUMO+2] are worked out for the complex [VO2(bmphp-sem)(H2O)]and the respective observed energies are as -6.5271, -6.00547, -5.60982,- 1.70760, -1.08327, -0.96327 eV, while the energy difference between [HOMO-LUMO], [HOMO-1‒LUMO+1] and [HOMO-2‒LUMO+2] are : 3.90222, 4.9222, 5.56383 eV, respectively. The electronic configuration of MO’s in shows the presence of paired electron in the HOMO justifying the diamagnetic nature of the investigated complex.
Energy of the frontier orbitals is related to the ionization energy (IE) and electron affinity (EA) derived from Koopmans’s theorem [59], and is given as:
-EHOMO=IE, ……... (i)
-ELUMO=EA, …..… (ii)
While as, IA and EA are related to absolute electronegativity (χabs) and absolute hardness [60] as given below:
χabs=(IE + EA)/2=(EHOMO+ELUMO)/2 ……(iii)
η=(IE - EA)/2=(EHOMO-ELUMO)/2 …….(iv)
Hard molecules have a large HOMO-LUMO gap, and soft molecules have a small HOMO-LUMO gap [61,62]. All the global reactive descriptors were calculated and are represented in Table S2. The molecular orbital structures with energy level description of the complex [VO2(bmphp-sem)(H2O)] is displayed in Figure 10.
Other important properties correlated with dipole moment and hardness are electrophilicity index (ω) and global softness (S) (equations shown below). The numerical assignments of ω and S are tabulated in Table S2.
ω=μ2/ 2η …...(v)
S=1/η ………(vi)
Atomic net charges: In order to determine delocalization of electron density between occupied Lewis-type orbitals and unoccupied non-Lewis NBOs (antibonding or Rydberg) (Table S3), which eables to find donor–acceptor interaction [63] atomic charges analysis of cis- [VO2(bmphp-sem)(H2O)] complex was fetched by the computational output data. The largest negative charges are located on the two oxygen atoms, O (7); (-0.93658e) and O (4); (0.62491e). Thus, the bond lengths of V-O (7) and V-O (4) see are different. The electron configuration of V is: [core] 4s(0.01)3d(3.60)4p(0.40)5s(0.22)4d(0.06) according natural bond orbital analysis. Thus, (17.9946) core electrons, (4.21610) valence electrons (on 4s, 3d, and 4p atomic orbitals) and 0.07309 Rydberg electrons (mainly on 5s, and 4d orbitals) give the 22.25980 electrons. This is in good approx with the calculated natural charge on vanadium atom (+0.74020) in the complex, which corresponds to the difference between 22.25980e and isolated V atom’s total electron count (23e). In addition, the five oxygen atoms and one nitrogen atoms have larger negative charge as shown in Table S3. Thus, the positive atomic charge upon the vanadium (V) was substantially reduced as a consequence of electron density donation from ligands. According to Table S3 and S4, the calculated electron density on the donor atoms of oxygen and nitrogen atoms is less than expected, while the electron density computed on the central ions is more than expected. These observations lay confirmation of electron transmission between donor atoms and central metal ion.
The natural atomic charges of cis-[VO2(bmphp-sem)(H2O)] obtained by two main methods of population analysis, Natural population analysis (NPA) and Mulliken population analysis are compared in Table S4. It is not an easy task to compare between Mulliken’s net charges and the atomic natural ones because of the difference in their backgrounds. It is further explained by considering the results showing surprisingly well pronounced differences between the two sets of charges. The observation indicates that the atoms namely C11, C32, C34, C41, C43, and all hydrogen’s including V are positively charged both in NPA and Mulliken analyses. The remaining atoms: C12-C15, C21, C22, C24-C26, C33, C37, and all oxygen namely O2, O3, O4, O5, O6, O7 and N5, N35, N36, N42, N44 are showing negative partial charges over them in both the analysis. While C23 bear positive charge in Mulliken analyses and negatively charge in NPA. The graphical representation of NBO and Mulliken charges of complex is given in Figure 11.
From the literature survey it has been suggested that the natural atomic charge is based on the theory of the natural population analysis. NBOs have been supposed as the linear combinations of the natural atomic orbitals. It may be added here that natural population analysis always follows Pauli’s exclusion principle [64].
Molecular electrostatic potential surface (meps) of complex: The molecular surface charge topography is a significant concept to localize the reactive behavior of a molecule with respect to different atomic constituents varying in nature. Under such question negative regions can be assigned as nucleophilic centers, whereas the positive regions are designated as potential electrophilic loci. It is a graphical presentation to display the molecular electrostatic potential pointing to molecular shape, size and electrostatic potential values and also can be related to various biochemical and physical phenomena. Figure 11 clearly presents the nature of various atoms in coordination environment. Five oxygen atoms and one nitrogen atom around vanadium is the region of most negative potential. The hydrogen atoms bear the region of maximum positive charge, especially the hydrogens of Schiff base ligand. The two hydrogens attached to the electronegative oxygen of the coordinated water molecule are most electropositive constituents. The green region in the MESP surfaces refers to a halfway potential between the two extremes red and dark blue colors. In other words it is the midpoint location between the extreme electronegative and electropositive sites.
From the combined experimental and theoretical approach dioxovanadium(V) complexes synthesized in this investigation are of the general composition [VO2(L)(H2O)] where LH=N-(4’-benzoylidene-3’- methyl-1’-phenyl-2’-pyrazolin-5’-one)-semicarbazone (bmphp-semH), N-(butyrylidene -3’-methyl-1’-phenyl-2’-pyrazolin-5’-one)-semicarbazone (bumphp-semH), N-(4’-iso-butyrylidene -3’-methyl-1’-phenyl-2’-pyrazolin- 5’-one)-semicarbazone (iso-bumphp-semH) or 3’-methyl-1’phenyl-4’- propiolidene-2’-pyrazolin-5’-one)-semicarbazone (mphpp-semH)]. Keeping in view the monomeric hexa-coordination of all the complexes, and the wellestablished octahedral structure of dioxovanadium(V) complexes, K[VO2(saliNH)( H2O)] and K[VO2(Clsal-iNH)(H2O)] involving N-isonicotinamidosalicylaldimines( aroylhydrazones) ligand (similar to LH in the present investigation), cis-octahedral structure (Figure 9) has been proposed for this class of complexes. X-ray crystallographic studies, which might confirm the proposed structures, could not be carried out, as suitable crystals were not obtained. The theoretical structural parameters have shown an excellent agreement with the experimental results and hence stem the fact of the reliability of the employed level of theory. In order to endure the investigative boost with respect to such class of compounds it is inferred that the target compounds might prove helpful in framing durable and long lasting materials.
The authors are thankful to Prof. K. N. Singh Yadav, Vice Chancellor, Rani Durgavati University, Jabalpur, Madhya Pradesh, India for encouragement. Analytical facilities provided by the Central Drug Research Institute, Lucknow, India and the Regional Sophisticated Instrumentation Centre, Indian Institute of Technology, Mumbai, India are gratefully acknowledged. Refer supplementary material for Tables S1-S4 and Figures S1-S4.