Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Research Article - (2015) Volume 4, Issue 1
In this work six 3-aryl-2-(substituted styryl)-4(3H)-quinazolinones derivatives were synthesized by the reaction of 3-aryl-2-methyl-4(3H)-quinazolinone (intermediate products) with different substituted aromatic aldehydes. Three intermediate products were synthesized by reacting 2-methyl-3,1-benzoxazin-4-one, which was initially prepared by cyclizing anthranilic acid using acetic anhydride, with three aromatic amines. Their structures were confirmed using IR, 1HNMR, 13CNMR spectroscopic methods and elemental microanalyses. The synthesized compounds were evaluated for their in vivo antimalarial activity against P. berghei. Four of the synthesized compounds (IIIc, IVa, IVb and IVf) exhibited activity against the parasite. Among these compound IVa was found to be the most active compound. Results of acute toxicity study showed that oral administration of the synthesized compounds in single doses (100, 250 and 500 mg/kg) had no adverse effects, indicating that the compounds have high safety margin and their LD50 is higher than 500 mg/kg.In general this study indicates that 4(3H)-quinazolinones derivatives are potential sources of lead compounds for anti malarial drugs.
<Keywords: 4(3H)-quinazolinones derivatives, In vivo, P. berghei, Anti-malarial
Malaria is one of the oldest recorded diseases in the world. Ancient Chinese and Sanskrit medical texts described its symptoms and Hippocrates referred to the disease in the 4th Century BC [1]. It is estimated to account for 300 million to 500 million illnesses and nearly 1 million deaths each year [2]. Malaria is a protozoal disease caused by parasites of the genus Plasmodium [3]. Four identified species of this parasite exist, which cause different types of human malaria, namely; Plasmodium vivax, Plasmodium falciparum, Plasmodium ovale and Plasmodium malariae [4,5]. Ethiopia had approximately 6% of malaria cases in the African Region in 2006 [6]. Almost 75% of the country’s land is malarious and an estimated 51 million (68%) of the people.
In the past 30 years, only one synthetic antimalarial drug (mefloquine) has been discovered. The other drug discovered during this period, artemisinin, is a natural product, whose medicinal properties were known for more than 2,000 years [7]. The sudden and dramatic resurgence of malaria in many countries have made synthetic efforts toward new antimalarial drugs very important [8]. Recently some synthetic compounds are reported to have potent antimalarial activity against different Plasmodium species.
Clinical trials conducted with fosmidomycin, chalcone analogue, naphtha quinone, acylation of the hydroxy moiety of atovaquone derivatives and aryl sulfonyl acridinyl derivatives were reported to have outstanding antimalarial activity [7,9-12]. Quinazolinones are versatile nitrogen heterocyclic compounds, diplaying wide applications including anticonvulsant, sedative, tranquilizer, analgesic, antimicrobial, anesthetic, anticancer, antihypertensive, antiinflammatory, antimalarial, diuretic and muscle relaxant properties [13-22]. Increased efforts in antimalarial drug discovery are urgent to develop safe and affordable new drugs to counter the spread of malaria parasites that are resistant to existing agents. Furthermore, quinazolinones substituted at 2 and 3- position play a pivotal role in the antimalarial activity [13].
Several bio-active natural products such as febrifugine and isofebrifugine contain quinazolinone moieties with potential antimalarial activity were also reported [11,12]. Therefore, 2,3-disubstituted-4(3H)-quinazolinones, are point of interest to seek for new drugs that act against the malarial pathogen in order to combat and reduce its tremendous prevalence. Hence, in this work compounds containing 4(3H)-quinazolinone moiety were designed to study their antimalarial activities. The simple synthesis and anti malarial results of these newly synthesized compounds are reported.
General
Melting points were determined in open capillaries using Buchi (B-540) melting point apparatus and are uncorrected. IR spectra were recorded in nujol SHIMADZU8400SP FT-IR spectrophotometer,1HNMR and 13CNMR spectra in CDCl3/CCl4 in Bruker Avance DMX-400 FT-NMR spectrometer using TMS as internal reference (chemical shifts in δ, ppm). Elemental microanalyses were performed on Perkin Elmer 2400 elemental analyzer. Elemental (C, H, N) analysis indicated that calculated and observed values were within + 0.4 of theoretical values. The purity of compounds was checked by thin layer chromatography on silica gel plate of 0.25 mm thickness using benzene: methanol (9:1) as a solvent system. Iodine chamber was used as a developing chamber. All the reagents used were AR grade.
General procedure for the preparation of 3-aryl-2-methyl- 4(3H)-quinazolinones (III a-c)
A mixture of acetanthranil II, (0.1 mol) and an equimolar amount of the appropriate aromatic amine was heated under reflux for 5-7 hrs. The dark sticky mass formed was cooled and recrystallized from ethanol.
2-Methyl-3-anilino-3H-quinazolin-4-one (IIIc)
Yield 69.6%; mp 187-189°C; IR (Nujol) (cm-1): 3264 (NH); 1675 (C=O) ; 1649 (C=N). 1HNMR (CDCl3/CCl4) δ ppm: 2.53 (s, 3H, CH3), 6 .56 (d, 2H, anilino C2,6 H), 6.8 (t, 1H, anilino C4 H), 7.1 (t, 2H, anilino C3,5 H), 7.35 (t, 1H, quinazolinone C6 H), 7.56 (d, 1H, quinazolinone C8 H), 7.67 (t, 1H, quinazolinone C7 H) and 8.06 (d, 1H, quinazolinone C5 H). 13CNMR (CDCl3/CCl4) δ ppm: 21.09 (1C, CH3), 113.13 (2C, anilino C2,6), 120.83, (1C, anilino C4), 121.91 (1C, quinazolinone C4a), 126.51 (1 C, quinazolinone C6), 126.59 (1C, quinazolinone C8 ), 126.65 (1C, quinazolinone C5), 129.27 (2C, anilino C3,5), 134.79 (1 C, quinazolinone C7), 145.78 (1 C, quinazolinone C8a), 146.61 (1C, anilino C1), 157.68 (1 C, 4(3H)-quinazolinone C2) and 161.12(1C, C=O).Anal. Calcd. forC22H16N4O3: C, 68.74; H, 4.20; N, 14.58. Found: C, 68.47; H, 4.47; N, 14.42.
General procedure for the preparation of 3-aryl-2-(substituted styryl)-4(3H)-quinazolinones (IVa-f)
A mixture of 3-aryl-2-methyl-4(3H)-quinazolinone IIIa-c, (10 mmol) and an equimolar amount of the appropriate aromatic aldehyde was allowed to react in the presence of fused sodium acetate by heating under reflux for 10-12 hrs. The solid products formed were filtered, washed with ethanol, dried and recrystallized from ethanol.
4-[(1E)-2-(3,4-dihydro-4-oxo-3-p-tolylquinazolin-2-yl) vinyl]-2-methoxy phenylacetate (IVa)
Yield 53%; mp 206-208°C; IR (Nujol) (cm-1): 1760 (C=O); 1670 (C=N); 1655 (C=O); 1220(C-O); 1119 (C-O); 1HNMR (CDCl3/CDCl4) δppm: 2.3 (s, 3H, p-tolyl CH3), 2.5 (s, 3H, acetateCH3), 3.8 (s,3H, O-CH3), 6.36 (d, 1H vinyl C1 H), 6.9-7.0 (m, 3H, methoxyphenyl C2,5,6 H), 7.2 (d, 2H, p-tolyl C3,5 H), 7.4 (d, 2H, p-tolyl C2,6 H)7.5 (t, quinazolinone C7 H), 7.76 (d, 2H, quinazolinone C6,8H), 7.9 (d, H, vinyl C2 H), 8.3 (d, 1H, quinazolinone C5 H); 13CNMR(CDCl3/CDCl4) δppm: 20.70 (1C, p-tolyl CH3), 21.36 (1C, acetate CH3), 55.78 (1C, methoxy CH3), 111.91 (2C, methoxyphenyl C2, 5), 119.97 (1 C, methoxyphenyl C6), 120.69 (1C, quinazolinone C4a), 120.98 (1C, vinyl C1), 123.15 (2C, p-tolyl C2,6), 126.64 (1C, quinazolinone C6), 127.20 (1C, quinazolinone C8), 127.32 (1C, quinazolinone C5), 128.39 (1C, p-tolyl C1), 130.60 (2C, p-tolylC3,5), 134.27 (1C, p-tolyl C4),134.50 (methoxyphenyl C1), 134.61 (1 C, quinazolinone C7), 138.93 (1C, vinyl C2), 139.38 (1C, methoxyphenyl C4), 147.76 (1 C, quinazolinone C8a), 151.16 (1C, methoxyphenyl C3), 151.76 (1C, quinazolinone C2), 162.36 (1C, quinazolinone C4) and 168.92 (1C, acetate C=O). Anal. Calcd. for C26H22N2O4 : C, 73.23; H, 5.20; N, 6.57. Found: C, 72.96; H, 4.91; N, 6.37.
4-[(1E)-2-(3,4-dihydro-4-oxo-3-p-tolylquinazolin-2-yl)vinyl] phenyl acetate (IVb)
Yield 66.3%; mp 199-201°C; IR (Nujol) (cm-1): 1760 (C=O); 1679 (C=O); 1654 (C=N); 1209 (C-O-C); 1109 (C-O-C); 1HNMR (CDCl3/ CDCl4) δppm: 2.3 (s, 3H,p-tolyl CH3), 2.5 (s, 3H, C=OCH3), 6.35 (d, 1H, vinyl C1 H), 7.04 (d, 2H, phenyl acetate C3,5 CH), 7.2 (d, 2H, p-tolyl C3,5 H), 7.36 (d, 2H, phenyl acetate C2,6 H) 7.4 (d, 2H, p-tolylC2,6H), 7.38 (t, quinazolinone C7 H), 7.77-7.80 (m, 2H, quinazolinone C6,8 H), 7.94 (d, H, vinyl C2 H), 8.3 (d, 1H, 4(3H)-quinazolinone C5 H); 13CNMR(CDCl3/CDCl4) δppm: 18.533 (1C, p-tolyl CH3), 21.47 (1C, phenyl acetate CH3), 120.16 (1C, quinazolinone C4a), 121.04 (2C, p-tolyl C2,6), 121.97 (2C, phenyl acetate C3,5), 126.47 (2C, yphenyl acetate C2,6), 127.28 (1C, quinazolinone C6), 127.38 (1C, quinazolinone C8), 128.43 (2C, p-tolyl C3,5), 128.80 (1C, quinazolinone C5), 130.57 (1C, p-tolyl C1), 133.13 (1 C, p-tolyl C4), 134.39 (1 C, quinazolinone C7), 134.32 (1C, phenyl acetate C1), 139.17 (1C, vinyl C2), 147.72 (1 C, quinazolinone C8a), 151.50 (1 C, quinazolinone C2), 151.54 (1C, phenyl acetate C4), 162.14 (1C, quinazolinone C4) and 168.65 (1C, acetate,C=O). Anal. Calcd. forC25H20N2O3: C, 77.04; H,5.08; N,7.07. Found: C, 76.82; H, 4.87; N, 7.21.
4-[(1E)-2-(3,4-dihydro-4-oxo-3-phenylquinazolin-2-yl) vinyl]-2-methoxy phenyl acetate (IVc)
Yield 48.7%; mp 221-223°C; IR (Nujol) (cm-1): 1759 (C=O); 1676 (C=O); 1637 (C=N); 1201 (C-O-C); 1119 (C-O-C);1HNMR (CDCl3/ CDCl4) δppm:2.3 (s,3H, –C=OCH3), 3.8 (s,3H, -O-CH3), 6.3 (d, 1H, vinyl C1 H), 6.8-7.0 (s and 2d,3H, methoxyphenyl C2,5,6 H), 7.35 (d, 2H, phenyl C3,5 H), 7.5 (t, 1H, quinazolinone C7 H), 7.56-7.63 (m,3H, phenyl C2,4,6 H), 7.8 (m, 2H, quinazolinone C6,8 H), 7.9 (d, 1H, vinyl C2 H), 8.3 (d, 1H, quinazolinone C5 H); 13CNMR(CDCl3/CDCl4) δppm: 20.61 (1C, -C=OCH3), 55.58 (1C, -O-CH3), 111.56 (1C, methoxyphenyl C2), 120.03 (1C, vinyl C1), 120.23 (1C, methoxyphenyl C6), 121.08 (1C, quinazolinone C4a), 123.15 (1C, methoxyphenyl C5), 126.53 (2C, phenyl C2,6), 127.27 (1 C, quinazolinone C6), 127.39 (1C, phenyl C4), 128.85 (1C, quinazolinone C8), 129.21 (2C, phenyl C3,5), 129.83 (1C, quinazolinone C5), 134.29 (1C, methoxyphenyl C1), 134.43 (1C, phenyl C1), 137.13 (1C, vinyl C2), 140.854 (1C, quinazolinone C8a), 147.79 (1C, methoxyphenyl C4), 151.20 (1C, methoxyphenyl C3), 151.27 (1C, quinazolinone C2), 161.93 (1C, quinazolinone C4) and 168.23 (1C, acetate C=O).Anal. Calcd. forC25H20N2O4: C, 72.80; H, 4.89; N, 6.79. Found: C, 73.04; H, 4.71; N, 6.59.
4-[(1E)-2-(3,4-dihydro-4-oxo-3-phenylquinazolin-2-yl) vinyl]phenyl acetate (IVd)
Yield 59.5%; mp 221-223°C; IR (Nujol) (cm-1): 1757 (C=O); 1679 (C=O); 1635 (C=N);1209 (C-O-C);1120 (C-O-C);1HNMR (CDCl3/ CDCl4) δppm:2.3 (s, 3H, -C=OCH3), 6.3 (d, 1H, vinyl C1 H), 7.05 (d, 2H, phenyl acetate C3,5 H), 7.30-7.35 (m, 4H, phenyl C3,5H and phenyl acetate C2,6 H), 7.50 (t, 1H, quinazolinone C7 H), 7.56-7.63 (m, 3H, phenyl C2,4,6H), 7.75-7.84 (m, 2H,quinazolinone C6,8 H), 7.9 (d, 1H, vinyl C2 H), 8.3 (d, 1H, quinazolinone C5 H);13CNMR (CDCl3/CDCl4) δppm: 21.08 (1C, acetate CH3), 120.00(1C, vinyl C2), 120.05 (2C, phenyl C2,6), 121.98 (1C, quinazolinone C4a), 126.51 (2C, phenyl acetate C3,5), 127.25 (1 C, quinazolinone C6), 127.42 (1C, phenyl C4), 128.75 (1C, quinazolinone C8), 128.79 (2C, phenyl acetate C2,6), 129.30 (2C, phenyl C3,5), 129.87 (1C, quinazolinone C5),133.02 (1C, phenyl C1), 134.42 (1C, quinazolinone C7), 137.07 (1C, phenyl acetate C1), 138.74 (1C, vinyl C2), 147.79 (1C, quinazolinone C4a), 151.31 (1C, phenyl acetate C4), 151.55 (1C, quinazolinone C2), 161.97 (1C, quinazolinone C4,C=O)and 168.56 (1C, acetate C=O). Anal. Calcd. forC24H18N2O3: C,75.38; H,4.74; N,7.33. Found: C, 75.52; H, 4.46, N7.60.
2-(2-Nitrostyryl)-3-phenyl-4(3H)-quinazolinone (IVe)
Yield 74%; mp 197-199°C; IR (Nujol) cm-1: 1677 (C=O); 1630 (C=N); 1603 and 1377 (NO2);1HNMR (CDCl3/CDCl4) δppm: 6.3 (d, 1H, vinyl C1 H), 7.28 (d, 1H, phenyl C4 H), 7.35 (d, 2H, phenyl C2,6H), 7.46 (t, 1H, quinazolinone C7 H), 7.48-7.57 (m, 3H,o-nitrophenyl C4,5,6 H), 7.60 (t, 2H, phenyl C3,5 H), 7.8 (d, 2H, quinazolinone C6,8 H), 7.9 (d, 1H, vinyl C2 H), 8.3 (d, 1H, quinazolinone C5 H), 8.4(d, 1H, o-nitrophenyl C3 H); 13CNMR (CDCl3/CDCl4) δppm: 121.19 (1C, vinyl C2), 124.63 (1C, quinazolinone C4a), 124.84 (C, o-nitrophenyl C3), 126.99 (2C, phenyl C2,6), 127.15 (1 C, quinazolinone C6), 128.74 (1C, phenyl C4), 128.80 (1C, quinazolinone C8), 129.35 (2C, o-nitrophenyl C6), 129.46 (2C, phenyl C3,5), 129.92 (1C, quinazolinone C5), 131.45 (1C, o-nitrophenyl C4), 133.06 (1C, quinazolinone C7), 134.51 (1C, o-nitrophenyl C1), 134.92 (1C, phenyl C1), 136.89 (1C, vinyl C2), 147.56 (1C, quinazolinone C8a), 148.47 (1C, o-nitrophenyl C2), 150.32 (1C, quinazolinone C2)and 161.82 (1C, quinazolinone C4,C=O). Anal. Calcd. for C22H15N3O3:C, l 71.54; H,4.09; N,11.38. Found: C, 71.82; H, 3.86; N, 11.67.
2-(2-nitrostyryl)-3-anilinoquinazolin-4(3H)-one (IVf)
Yield 92.1%; mp 201-203°C; IR (Nujol) (cm-1): 3254 (NH); 1682 (C=O); 1630 (C=N); 1603 and 1377 (NO2); 1HNMR (CDCl3/CDCl4/ MeOD-d4) δppm: 6.85 (d, 1H, vinyl C1 H), 7.02 (t, 1H, anilino C4 H), 7.27 (t,3H, anilino C3,5H and o-nitrophenylC4H), 7.35 (s, 1H, anilino NH), 7.48-7.57 (m,3H, o-nitrophenyl C4,5,6 H), 7.6 (t, 1H, quinazolinone C7 H), 7.68 (d, 1H, o-nitrophenyl C5 H), 7.80-7.84 (m, 2H, quinazolinone C6,8 H), 8.05 (d, 1H, vinyl C2 H), 8.3 (d, 1H, quinazolinone C5 H), 8.6 (d, 1H, o-nitrophenyl C3 H).Anal. Calcd. for C15H13N3O: C, 71.70; H, 5.21; N, 16.72. Found: C, 71.57; H, 5.52; N, 16.91.
Experimental animals
Swiss albino mice of both sexes, weighing 25-35 g and aged 6-8 weeks purchased from Ethiopian Health and Nutrition Institute were used in the study. The mice were acclimatized to the laboratory conditions (temperature of 23-25°C with average relative humidity of 60%) for a period of 7 days before use. The mice were housed in standard cages and maintained on standard pelleted diet and water.
The Plasmodium berghei parasite
The rodent malaria parasite, P. berghei ANKA ((chloroquine sensitive) strain, obtained from the Biomedical lab at the Department of Biology, Faculty of Science, Addis Ababa University, Ethiopia was used to infect the mice for a four-day suppressive test.
In vivo antimalarial activity
In vivo antimalarial activity test of the synthesized compounds was performed using a 4-day standard suppressive test [23].On day 0, the test mice were injected with 0.2 ml of 2 × 107 parasitized erythrocytes, (P. berghei ANKA strain) intravenously. After 2 hr, the infected mice were weighed and randomly divided into five groups of five mice per cage. Groups 1, 2 and 3 received the synthesized compounds at 20 mg/ kg and 40 mg/kg dose levels and served as treatment groups [24]. Group 3 received the vehicle (7% Tween 80, 3% ethanol in water) and served as negative control. Group 4 received the standard drug chloroquine phosphate (20 mg/Kg) and served as positive control [25].
On days 1 to 3, animals in the experimental groups were treated again (with the same dose of the synthesized compound and same route daily) as in day 0. On day 4 (i.e. 24 hr after the last dose or 96 hr post-infection), blood smear from all test animals was prepared using Giemsa stain. Level of parasetimia was determined microscopically by counting 4 fields of approximately 100 erythrocytes per field. The difference between the mean value for the negative control group (taken as 100%) and those of the experimental groups was calculated and expressed as percent suppression or activity.
Untreated control mice typically die in about one week after infection [26]. For treated mice the survival-time (in days) was recorded and the mean survival time was calculated in comparison with that of the negative group [27].
In vivo acute toxicity
Oral acute toxicity study was done for the synthesized compounds. Four groups of mice, each group consisting of six male mice were used for testing acute toxicity. The mice in each group were fasted over night and weighed before test. Test compounds were dissolved in 70% Tween 80 and 30% ethanol. This solution was further diluted 10-fold with sterile distilled water to give a stock solution containing 7% Tween 80 and 3% ethanol [28]. Mice in groups one, two and three were given 100, 250 and 500 mg/kg/day of the synthesized compounds respectively with a maximum dose volume of 1 ml/100 g of body weight orally while mice in the control group (group four) were treated with the vehicle. After administration of the substance food was withheld for a further 2 hr period [29]. Toxicity signs such as changes in skin, eyes (blinking), tremors, convulsion, lacrimation, muscle weakness, sedation, urination, salivation, diarrhea, lethargy, sleep, coma and also death were observed for 72 hrs. Twenty-four hours later, the % mortality and weight of mice in each group and for each test compound at each dose level were recorded [30].
Data analysis
Results of the study were expressed as mean ± standard deviation. Statistical significance for suppressive test was determined by one-way ANOVA at 95% confidence limits (p=0.05). Data on body weight and survival time were analyzed. All the data were analyzed using Microsoft office excel 2007.
Percentage parasitaemia and percentage suppression were calculated using the following formulae:
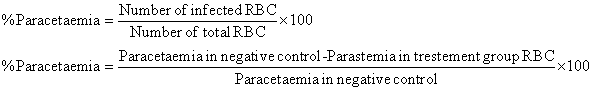
Chemistry
The required starting 2-methyl-3,1-benzoxazin-4-one II, was synthesized from anthranilic acid I and acetic anhydride [31]. 2-Methyl-3-aryl-3H-quinazolin-4-ones IIa-c,was prepared according to reported methods [24]. A mixture of acetanthranil II, (15.67g, 0.1 mole) and an equimolar amount of the appropriate aromatic amine was heated under reflux for 5-7 hrs. The 3-aryl-2-(substituted styryl)- 4(3H)-quinazolinones IVa-f, were prepared by Perkins condensation of IIIa-c with substituted aromatic aldehydes [32]. A mixture of 3-aryl-2-methyl-4(3H)-quinazolinone IIIa-c, (10 m mole) and an equimolar amount of the appropriate aromatic aldehyde was allowed to react in the presence of fused sodium acetate by heating under reflux for 10-12 hrs.
A series of novel 2,3-disubstituted styryl-4(3H)-quinazolinone derivatives IVa-f were prepared in good yields. The synthetic routes to these compounds are given in Figure 1. The structures of the compounds were verified based on data from elemental analysis, IR, 1HNMR and 13CNMR spectral studies.
IR studies
The summarized characteristic stretching and bending IR vibration frequencies of important functional groups are discussed below. The IR spectrum of compound IIIc showed a characteristic absorption band at 3264 cm-1(-NH), 1675 cm-1(>C=O) and at 1649 cm-1(>C=N).The appearance of a band at 1760 cm-1 (>C=O) is evident for the presence of acetate in compound IVa and other characteristic absorption bands appeared at 1670 (>C=N),1655 cm-1(>C=O).The other bands appeared at 1220 and 1119 cm-1 were attributed to the ester group. Characteristic IR absorption bands of compound IVbappeared at 1760 cm-1(>C=O), 679 cm-1(>C=O), 1654 cm-1(>C=N), 1209 and 1109 cm-1(C-O-C).Compound IVc showed a sharp absorption band that at 1759 cm-1(>C=O), 1676 cm-1(>C=O), 1637 cm-1(>C=N), 1201 and 1119 cm-1(C-O-C).Where as IR absorption bands of compound IVd appeared at 1757 cm-1(>C=O), 1679 cm-1(>C=O) 1635 cm-1(>C=N), 1209 and 1120 cm-1(C-O-C).The characteristic absorption bands ofIVe appeared at 1677 cm-1(>C=O), 1630 cm-1(>C=N), 1603 and 1377 cm- 1(-NO2).On the other hand the IR spectrum of compound IVf showed a sharp absorption band at 3254 cm-1(-N-H),1682 cm-1(>C=O), 1630 cm-1(>C=N).
NMR studies
The 1HNMR spectrum of the compound IIIc showed a doublet at δ 8.15 ppm which is representative for the C5proton of 4(3H)- quinazolinone. A doublet at δ 6.56 ppm, and a multiplate at δ 6.8 ppm and δ7.1 ppm indicated the presence of phenyl ring. The appearance of three singlets at δ 2.3, δ 2.5 and δ 3.8 ppm in the1HNMR spectra of Iva clearly indicates the p-tolyl, acetate and methoxy moieties respectively. The doublets at δ 6.4 ppm and δ 7.9 ppm confirms for the presence of styryl vinilic protons. The peaks characteristic to the 4(3H)-quinazolinone moiety appeared at δ 8.3 ppm, δ 7.5 ppm, and 7.8 ppm.The disappearance of signals corresponding to the protons of the methoxy group and appearance of multiplets for aromatic proton in the range of δ 6.4 ppm-8.3 ppm clearly indicate the formation of IVb. In the1HNMR spectrumof compound IVc peaks that appeared at δ 7.35 ppm and δ 7.56-7.63 ppm denotethe five phenyl protons.The absence of proton signals of the methoxy groupin the 1HNMR spectrum of IVd and the appearance of all the other peaks provedthe formation of IVd.The appearance of an unusual peak at δ 8.4 ppm in the 1HNMR spectrum of compound IVe is due to the proton ortho to the NO2. The presence of a singlet peak at 7.4 ppm in the 1HNMR spectrum of compound IVf is due to the NH group and other peaks at δ 7.02 ppm, δ 7.27 ppm,δ 7.48-7.57 ppm, δ 7.68 ppmand δ 8.6 ppm were attributed to the nine aromatic protons of the anilino and the o-nitrostyryl groups.
Another strong support for the structures of IIIc, IVa, IVb, IVc, IVd, and IVe is the 13C NMR spectra. The 13C NMR spectra of compounds were taken in CDCl3/CCl4 and the signals obtained were all in a good agreement with the proposed structures. The carbonyl and imine carbons of quinazolinone ring are resonates at about d 170.0 ppm and 160.1 ppm respectively for all of the compounds. In addition to this the 1H-1H COSY, DEPT-135, HMBC and HSQC spectra of compound IVa were observed.
In vivo antimalarial activity
To ascertain their antimalarial activity, compounds IIIc, IVa, IVb, IVc, IVd, IVe and IVf were assayed in vivo against P. berghei, a rodent malaria parasite, using a 4-day standard suppressive test [26]. The synthesized compounds were given at dose levels of 20 mg/kg and then 40 mg/kg to see if there is a dose-response relationship (Table 1). A dose of 20 mg/kg was considered to be the initial low dose based on previous works done for other synthesized compounds [27].
| Test substance | Dose mg/kg | % Parasitaemia | % Suppression | Mean survival time (Days) |
| IIIc | 20 | 58.2 ± 0.2 | 15.5 | 8.5 ± 0.6 |
| 40 | 27.4 ± 1.1 | 70.7 | 9.0 ± 0.5 | |
| IVa | 20 | 49.8 ± 0.2 | 27.7 | 9.2 ± 0.6 |
| 40 | 20.2 ± 2.0 | 78.4 | 9.8 ± 0.4 | |
| IVb | 20 | 61.6 ± 0.1 | 10.6 | 8.5 ± 1.0 |
| 40 | 36.9 ± 2.0 | 60.6 | 9.3 ± 0.6 | |
| IVc | 20 | 41.8 ± 0.2 | 39.3 | 9.0 ± 0.8 |
| 40 | 86.8 ± 0.6 | 7.3 | 7.7 ± 0.6 | |
| IVd | 20 | 48.8 ± 0.1 | 29.2 | 7.6 ± 0.5 |
| 40 | 89.2 ± 2.5 | 4.7 | 6.7 ± 1.2 | |
| IVe | 20 | 44.5 ± 0.1 | 35.4 | 9.7 ± 0.5 |
| 40 | 90.6 ± 1.7 | 3.2 | 7.3 ± 0.5 | |
| IVf | 20 | 51.8 ± 2.2 | 24.8 | 8.3 ± 0.6 |
| 40 | 26.6 ± 0.5 | 71.6 | 8.5 ± 0.6 | |
| NC (20) | 1ml/100 g | 68.9 ± 0.1 | 0.0 | 6.4 ± 0.5 |
| NC (40) | 1ml/100 g | 93.6 ± 0.1 | 0.0 | 6.6 ± 0.5 |
| Chloroquine | 20mg/kg | 0.0 | 100 | ND |
*Values are M ± SD, P<0.05, NC: Negative control, ND: No death recorded over the experimental period
Table 1: Antiplasmodial activities of the synthesized compounds at 20 mg/kg and at 40 mg/kg*
One-way ANOVA of the percent suppression of the negative control with groups that received the test compounds revealed that only compounds IVc and IVe showed statistically significant percent suppression at 20 mg/kg at 95% confidence limits (P=0.05). On the other hand among the groups that received the test compounds at a dose level of 40 mg/kg the result revealed that compounds IIIc, IVa, IVb and IVf exhibited statistically significant percent suppression. In general, the compounds IIIc, IVa, IVb, and IVf having secondary amine group,2-nitrostyryl group and methyl group of p-tolyl substituent showed very promising activity with percent suppression of 70.7, 78.4, 60.6 and 71.6 at 40 mg/kg in vivo against P. berghei as compared to the standard drug chloroquine phosphate. Whereas the rest of the compounds showed moderate and lower activity against P. berghei as compared to the standard drug chloroquine phosphate as shown in Table 1. None of the compounds were as equipotent as the standard drug chloroquine phosphate. The data also revealed that the activity of compound IVa>IVf>IIIc>IVb. Therefore, it can be inferred that presence of polar and non-planar substituents imparts much towards antimalarial power of these compounds.
In vivo acute toxicity
A preliminary toxicity test was conducted to assess the acute lethal, physical and behavioral effects of the synthesized compounds after oral administration to mice. The toxicity study was designed to verify whether the synthesized compounds are safe to be used for subsequent experiments or not. Acute toxicity study was performed for the two most active compounds IVa and IVf that showed good activity when given at both dose levels. Oral administration of the two relatively active compounds in doses of 100, 250 and 500 mg/kg did not produce any significant acute toxic effects on the experimental mice.
Gross behavioral and physical observations like hair erection, urination, muscle weakness, sedation and convulsion, reduction in feeding activity in the test mice were used as indicators of acute toxicity effects. The test mice monitored once daily for 14 days did not show any of the signs of toxicity mentioned. None of the test animals died within the first twenty-four hour. The data indicated that the test compounds did not show any significant change in weight and the slight variation observed was not found to be dose dependent (Table 2).
| Test substances | Dose mg/Kg | Wt. before test | Wt. after test |
| IVa | 100 | 26.6 ± 1.3 | 25.7 ± 1.5 |
| 250 | 23.0 ± 1.3 | 25.7 ± 1.1 | |
| 500 | 25.0 ± 1.4 | 25.4 ± 1.8 | |
| IVf | 100 | 27.1 ± 1.4 | 29.4 ± 1.7 |
| 250 | 26.1 ± 1.7 | 26.5 ± 1.4 | |
| 500 | 28.3 ± 1.6 | 29.5 ± 1.8 | |
| NC | 1ml/100g | 28.2 ± 1.7 | 27.3 ± 1.5 |
* Key: Values are M ± SD
Table 2: Result of acute toxicity studies*
The results of the study showed no adverse effects for the synthesized compounds indicating that the median lethal dose (LD50) of synthesized compounds could be much higher than 500 mg/kg/day for mice through oral route. The results indicated that test compounds proved to be non-toxic and well tolerated by the experimental animals up to 500 mg/kg.
In this study, a number of structurally related 4(3H)-quinazolinone derivatives were synthesized. Structures of the synthesized compounds were confirmed by various spectroscopic methods and elemental microanalyses. All the target compounds were efficiently synthesized in good yield and purity.
One representative intermediate and six target compounds were screened for their antimlarial activity in P. berghei infected mice. The result revealed that four of the synthesized compounds IIIc, IVa, IVb and IVf were having a promising antimalarial activity (60.6-78.4% suppression of the parasitaemia), but none of the compounds were as active as the reference standard drug, chloroquine, in the doses tested. Compounds IVc and IVe showed significant suppression at initial low dose (20 mg/kg) while compound IVd showed a very week activity at Oral acute toxicity profile was determined for the two most active compounds, IIIc and IVa. The result revealed that oral administration of the synthesized compounds in single doses (100, 250 and 500 mg/kg) had no adverse effects, indicating that the compounds may have high safety margin and their LD50 could be higher than 500 mg/kg. Since the 4(3H)-quinazolinone derivatives have not been used as antimalarial agents the existence of resistance against these compounds is barely true. Therefore these 4(3H)-quinazolinone derivatives are expected to have activity against plasmodium species resistant to existing drugs.
In this work only limited numbers of compounds were synthesized and their antimalarial activity was tested. Thus more compounds should be synthesised and tested to exploit the possible most active 4(3H)-quinazolinone derivatives. Additional toxicity studies need to be done to prove the sub-acute and chronic safety of the synthesized compounds. The antimalarial mechanism of action of 4(3H)- quinazolinone derivativesneed to be determined and docking study be performed.
We are very grateful to our college staff members for unreserved guidance and constructive suggestions and comments from the stage of proposal development to this end. We would like to thank Addis Ababa University for supporting the budget which required for this research.