
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Review Article - (2023)Volume 6, Issue 1
This experiment uses the concept of diversity-oriented synthesis as the starting point, combined with the ionic liquid carrier developed by the previously mentioned green chemistry (ion-liquid support) and microwave assisted organic synthesis (microwave assisted organic synthesis) to rapidly synthesize complex and diverse small molecule compounds. Pictet-Spengler reaction into 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole derivatives using an boc removed tryprophan extended Pictet-Spengler reaction with the alkyne and cyclization cyanide, so the synthesis strategy of the compound is drawn up as, the ionic liquid compound, Then start the extended Pictet-Spengler reaction under acidic conditions to get 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole derivatives, in order to fully meet the green. Therefore, the original reaction conditions in this laboratory were improved to use a synthetic method that does not pollute the environment. The conditions of the reaction can achieve the most efficient and non-polluting environment.
Nuclear magnetic resonance; Tetrahydro-β-carboline; Bicyclohexylene amine; Trifluoroacetic acid
Tetrahydro-β-carboline plays a very important role in natural substances, in many natural products contain tetrahydro-β-carboline, the following four compounds are all very common example written in many studies found that derivatives of tetrahydro-β-carboline have some special properties. The biological activity of compound is an antagonist of serotonin 2B receptor agent, and serotonin is a very important neurotransmitter in the central nervous system, if when serotonin is out of whack, it can lead to symptoms like anxiety, depression, and migraines, which symptoms can be treated with receptor antagonists; and the compound Tadalafil has also been reported to inhibition of phosphodiesterase type 5 associated with erectile function in men with cardiovascular disease (PDE5) 40 receptor; the reason is that the main function of PDE5 is to convert cyclic Guanosine Monophosphate (cGMP) is hydrolyzed to form Guanosine Monophosphate (GMP), and cGMP can be regarded as flat smooth muscle relaxants are stimulated by another neurotransmitter, Nitric Oxide (NO), causing to relax the corpus cavernosum and achieve erection, if PDE5 is inhibited, it can make cGMP reaches a certain concentration in the tissue, and then promotes the erection of the penis, as shown in Figure 1. Many studies have shown that molecules with tetrahydro-β-carboline can have good anticancer activity. This experiment uses the concept of diversity-oriented synthesis as the starting point, combined with the ionic liquid carrier developed by the previously mentioned green chemistry (ion-liquid support) and microwave assisted organic synthesis (microwave assisted organic synthesis) to rapidly synthesize complex and diverse small molecule compounds.
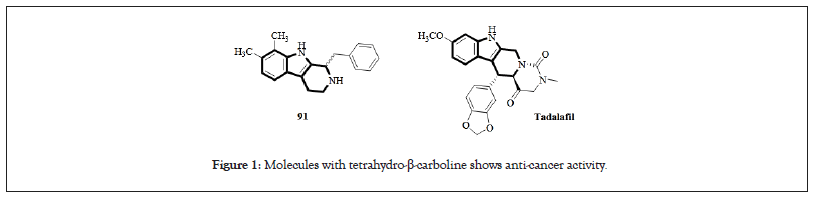
Figure 1: Molecules with tetrahydro-β-carboline shows anti-cancer activity.
Natural product discovery by random screening of both extracts derived from cultured bacteria often suffers from high rates of redundant isolation, making it ever more challenging to identify novel biologically interesting natural products. Here we show that homology-based screening of soil metagenomes can be used to specifically target the discovery of new members of traditionally rare, biomedically relevant natural product families. Phylogenetic analysis of oxy-tryptophan dimerization gene homologs found within a large soil Deoxyribonucleic Acid (DNA) library enabled the identification and recovery of a unique tryptophan dimerization biosynthetic gene cluster, which we have termed the bor cluster. When heterologously expressed in Streptomyces albus, this cluster produced an indolotryptoline antiproliferative agent with CaMKIIδ kinase inhibitory activity (borregomycin A), along with several dihydroxyindolocarbazole anticancer/antibiotics (borregomycins B-D). Similar homology-based screening of large environmental Deoxyribonucleic Acid (DNA) libraries is likely to permit the directed discovery of new members within other previously rare families of bioactive natural products.
1,2,3,4-Tetrahydro-β-carbolines (THβCs), a compound class within the indole alkaloids, consist of a variety of both simple and complex natural and synthetic compounds [1]. These compounds possess a vast spectrum of biological activities and their use in novel pharmacological applications is under constant study, as the THβC structure is present in drugs currently available on the market, drug candidates under development and many other pharmacologically interesting compounds [2-17].
One synthetically interesting subgroup among the THβCs is the optically active THβCs with C1-substitution. A stereocenter at C1 is a typical feature in natural THβCs and establishment of this stereocenter has received plenty of attention. C1 substituted THβCs have a wide variety of pharmacological properties, including PDE5 inhibitory, antimalarial, antiviral and antitumor activities. This review summarizes the methods to create the C1 stereocenter and describes the pharmacological activity of simple C1 substituted THβCs. This review offers a welcome update to a previous review discussing β-carbolines [18]. Furthermore, this is the only review focusing on C1-substituted THβCs and this focus allows covering these compounds in more detail. β -carboline alkaloids are an important group of natural and synthetic indole alkaloids which all bear the common feature of a tricyclic pyrido[3,4-b]indole ring structure. The first β-carboline alkaloid recognized was harmalin, originally isolated in 1841 from Peganum harmala, also known as Syrian rue [19]. The occurrence of β-carbolines in nature is widespread, presumably due to their simple biogenesis from tryptamine (or tryptophan), and today β-carbolines have been isolated from various plant families, fungi, animal tissues and marine sources. The fully aromatic members of this group are named β-carbolines (βCs) 1, whereas the members with partially saturated C-rings are known as 3,4-dihydro-β-carbolines (DHβCs) 2 and 1,2,3,4-tetrahydro-β-carbolines (THβCs) (Figure 2).
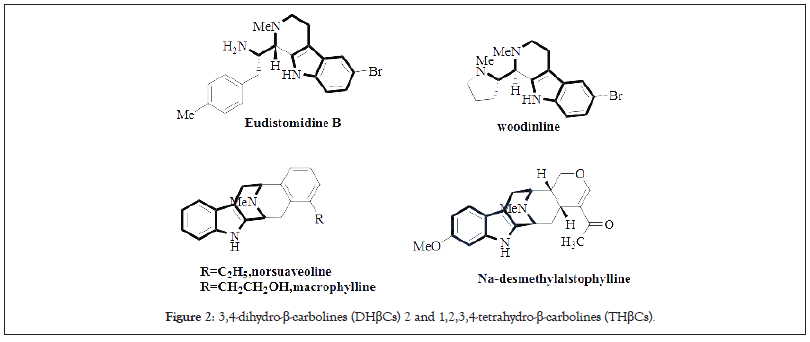
Figure 2: 2: 3,4-dihydro-β-carbolines (DHβCs) 2 and 1,2,3,4-tetrahydro-β-carbolines (THβCs).
The best known natural THβCs have been isolated from Peganum harmala and Pausinystalia yohimbe (formerly Corynathe yohimbe). Yohimbe alkaloids encompass such pharmacologically interesting natural products as yohimbine and its isomers, reserpine and ajmalicine the latter two being currently used as antihypertensive drugs. Harmala alkaloids include various β-carbolines including the THβCs tetrahydroharmine (an active ingredient in yaje, or ayahuasca, a hallucinogenic brew prepared from the Amazonian plant Banisteriopsis caapi), tryptoline, harmicine and pinoline (a melatonin metabolite produced in the pineal gland) [20]. Today, the most important synthetic compound encompassing the THβC structure is tadalafil, which has reached almost $2 billion annual sales in the treatment of erectile dysfunction under the brand name Cialis [21]. Tadalafil is also used for pulmonary arterial hypertension treatment under the brand name Adcirca.
Tetrahydro-β-carboline library A mixture of 1-methyiimidazole 92(0.28 g,0.04 mmol.1 equiv)and 1-bromoethanol 93 (5.0 g, 0.04 mmol,1 equiv) in 50 ml round bottom flask equipped with a drting tube was placed in a 250 ml beaker containing 50 ml of water. The reaction mixture was heated intermittently (360 W 60 s+50 s+30 s) in the open vessel microwave oven. The temperature of water bath was below 70°C during the course of the reaction. The pale brown oil solidified on cooling and was washed by cold ether (10 ml × 3). The product was obtained as a pale yellow compound. A mixture of 1-(2-hydroxyethyl)-3-methylimidazolium bromide (8.2 g, 0.04 mmol, 1 equiv) 94 and NaBF4 (8.8 g, 0.08 mmol, 2.0 equiv) and dry acetonitrile (25 m) was heated to reflux with stirring for 25 hours under nitrogen. Upon cooling,the white precipitate was filtered of and wased with acetonitrile (10 ml × 2). The organic filtrate was concentrated in vacuum to give liquid compound. compound (1.0 g, 4.7 mmol, 1.0 equiv) dissolved in acetonitrile was added to Fmoc-L-tryptophane compound (5.0 g,11.75 mmol, 2.5 equiv) or, in the presence of N,N 2-dicyclohexylcarbodiimide (0.18 g,0.30 mmol, 1.8 equiv) and N,N 2-Dimethylamino Pyridine (DMAP) (cat.amount). The reaction was stired in room temperature for 10 hours. If under subsequently irradiated microwave assisted on open vessel system,reaction time can redused to 20 minute .After reaction was finished , the suspensible Dicyclohexyl Urea (DCU) was filtered through filter paper. The reaction crude were precipitated by slow addition of cold ether and precipitated product compound IL-04 was filtered through fritted funnel and used cool ether to remove impurity ,than used acetonitrile dissolved reaction productand dried acetonitrile to obtain pale yellow soild compound IL-01. Compound IL-04 (1 g, 01.63 mmol,1 equiv) was dissolved by 10% piperidine in solvent(H2O:Isoprapanol=1:1). The reaction was stired in room temperature for 30 hours or assisted by CEM microwave oven (70°C 10 bar, 90 s). The reaction crude were used the same purification method to removed reaction impurity.The deprotection product were obtained pale yellow soild compound IL-05. A mixture of compound IL-05 and unsturated alkyne compound was dissolved by solvent (H2O:Isoprapanol=1:1) in 5 ml CEM reactor.
The vial was sealed, immediately irradiated at 90°C (bymodulation of the power) for 10 min, then added 0.5 ml acetic acid stired in room temperature for 10 min . The reaction crude were used the same purification method to removed reaction impurity. The deprotection product were obtained pale yellow soild compound IL-07. Compound IL-07 (0.2 g,0.48 mmol,1 equiv),4-Chlorophenyl isocyanate (0.23 g,1.50 mmol, 3 equiv) and potassium carbonate(0.11 g,0.8 mmol, 8 equiv) were added.The reaction was stired in room temperature for 8 hoursor immediately irradiated at 110°C (by modulation of the power) for 10 ~ 15 min. The reaction mixtures were filtered through filter paper to remove K2CO3. The reaction crude was precipitated by slow addition of cold ether. Collected the cool ether. The solvent was removed in vacuo to analyse 1H NMR of the concentrated ether layerd. Column chromatography (4:1 hexane/EtOAc, Rf=0.3) of the crude residue yielded compound as a colorless oil. When same reaction situation and purification method, but reaction reagent changed to isothiocyanate, such as n-butyl isothiocyanate derivative compound.
Soluble ionic liquids have recently been used as supports for catalyst/reagent immobilization and synthesis in homogeneous solution phase. 1-methylimidazole (1-methylimidazole) compound and 1-bromoethanol (1-bromoethanol) compound, etc, it is obtained by adding the equivalent amount to the reaction vessel and reacting successively for 60 seconds, 50 seconds, and 30 seconds under the assistance of microwave to compound 1-(2-hydroxyethyl)-3-methlimidazolium bromide, with glacial diethyl ether. After cleaning, the product was added to two equivalents of sodium borofluoride for an ion exchange step using acetonitrile. As a solvent reflux for 24 hours, and then use gravity filtration to filter out sodium bromide and excess tetrafluoroethylene sodium boride was reprecipitated with glacial ether to give the product, 1-(2-hydroxyethyl)-3-methl imidazolium tetrafluoroborate compound, and finally washed with glacial ether. Combine the synthesized ionic liquid carrier compound with the tryptamine group whose amine group is protected by Fmoc Acid (Fmoc-L-tryptophane) uses acetonitrile as a solvent in the coupling reagent bicyclohexylene amine and catalyst N,N'-dimethylaniline pyridine under the esterification reaction to generate compounds IL-04, its reaction mechanism after the reaction finishes, first the by-product Dicyclohexylurea (DCU) gravity filtration is filtered out, after removing a large amount of B Nitrile, then completely precipitate the product with a large amount of ice acetonitrile, and then remove the by-product by suction filtration and impurities, and finally dissolve the precipitate with acetonitrile and collect it to obtain the product; this step takes more than one day to stirr at room temperature. If you use a household microwave or a focused micro wave reaction furnace can shorten the reaction time to a few minutes, and the obtained product can be detected by Hydrogen Nuclear Magnetic Resonance (1H NMR) spectroscopy to monitor. Then compound IL-04 was reacted with 10% piperidine in acetonitrile at room temperature for three minutes. If you use a household microwave or a focused microwave reactor for ten minutes, the reaction time can be shortened to 3 minutes The compound IL-05 (as shown in Figure 3) is obtained by removing the Fmoc protecting group within 1 minute. This time, the purification of the reaction is the same as the previous method, and the reaction machine is used to capture piperidine. The hydrogen of the fluorene benzyl group, and then via the transfer of electrons, the compound IL-05, the dioxide carbon and 9-methylene-9H-fluorenece were monitored by Hydrogen Nuclear Magnetic Resonance (1H NMR) spectroscopy. Compound IL-05 and unsaturated alkyne compound were stirred with acetonitrile as solvent at room temperature. Stir for 12 hours to make it carry out Michael addition to obtain the intermediate product enamine compound IL-06, if compound IL-05 will be carried out with unsaturated alkyne under microwave system Continuous Emission Monitoring (CEM). Perform Michael addition reaction for 5 to 10 minutes (90°C, 5 bar) to get the intermediate product compound IL-06, this step cannot identify the intermediate product with NMR spectrum, so directly add 3 to 5 drops of trifluoroacetic acid (Trifluoroacetic acid) to the unpurified intermediate product IL-06. Stir for ten minutes, in the indole (indole), will provide electrons to the compound IL-06, and rise to another. An intermediate product compound IL-08 can be obtained through a proton transfer at the end compound IL-07. Changing to less polluting vinegar in consideration of not polluting the environment acid but its acidity is not strong enough so it needs to be increased to 5% to 10% by volume and the reaction is dissolved. The solvent was also changed from acetonitrile to a 2:1 mixed solvent of deionized water and isopropanol, and stirred at room temperature for 10 minutes. Make it perform extended Pictet-Spengler reaction, and then use the aforementioned purification method to carry out. The product compound IL-07 can be obtained after purification, and the optimization process is shown in Table 1. To increase the variability of derivatives in this step, try to utilize many different alkynes for the reaction, but only in the α, β unsaturated alkyne can be stretched Pictet- Spengler reaction, as in methyl propiolate, ethyl propiolate, 3-butyn-2-one, propiolic acid (propiolic acid), dimethyl but-2-ynedioate, diethyl but-2-ynedioate, ethyl 3-phenylpropiolate can be completed into the desired stretchable Pictet-Spengler reaction; and in phenylacetylene (Phenylacetylene), Propargyl alcohol , 2-propargylamine (Prop-2-yn-1-amine), etc. cannot complete the expected reaction. The main reason should be because of such alkynyl groups are not electron-deficient enough to effectively generating the intermediate product enamine, so there is no way to complete the reaction. First compound IL-07, using 5 equivalents of triethylamine and 3 equivalents of isocyanate or 3 equivalents of thioisocyanate acetonitrile is used as solvent reaction to form compound, and this reaction is compound IL-07 first reacts with isocyanate (or thioisocyanate) to form urea (or thiourea) intermediate product compound, and then triethylamine to remove the hydrogen of tetrahydro-β-carboline, at this time urine another secondary amine of the prime (or thiourea) intermediate compound will attack the ester of the sub-liquid product, the product is cut directly from the ionic liquid to obtain the crude product and the starting ionic liquid carrier, Under the double consideration of time and environment, the conditions of the experimental reaction and the addition of the reaction make some adjustments to the reagent, the step of removing the ionic liquid can be made more effective under the adjustment of Table 1 effectively reduce the pollution caused by the experiment. And when potassium carbonate was used as the reagent, it was also found that there would be a difference with the removal of the polymer carrier. In the same situation, when triethylamine is added, because the added isocyanate is over equivalent, so excess isocyanate will undergo self-coupling into urea under the condition of base catalysis, and its reaction as shown in Scheme 3-4, the presence of urea will affect the difficulty of subsequent purification, but if potassium carbonate is used as a reagent, the amount of urea generated will be greatly reduced.
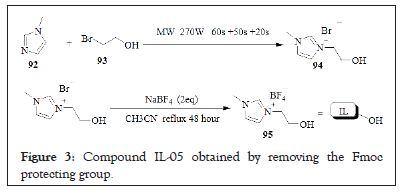
Figure 3: Compound IL-05 obtained by removing the Fmoc protecting group.
| Entry | R1 | R2 | Yield |
|---|---|---|---|
| 5 | COOMe | H | 77 |
| 6 | COOEt | H | 78 |
| 7 | COMe | H | 81 |
| 8 | COOH | H | 84 |
| 9 | COOMe | COOMe | 88 |
| 10 | COOEt | COOEt | 90 |
| 11 | COOEt | Ph | very rare |
| 12 | Ph | H | - |
| 13 | CH2OH | H | - |
| 14 | CH2NH2 | H | - |
Table 1: Purification and optimization process of compound IL-07.
The wide range of ionic liquid supports available makes their use compatible with most common chemistries. The solubility properties of these ionic liquid supports can be tuned by the variation of cations and anions to make them phase separate from less polar organic solvents and aqueous media. The ionic-liquid-supported species can therefore be purified from the reaction mixture by simple washings. Ionic-liquid-supported catalysts and reagents have been prepared and used, and they are easily recovered and reused. Parallel and combinatorial libraries of small molecules have been synthesized. Ionic-Liquid-Supported Synthesis (ILSS) has been applied to the preparation of oligopeptides and oligosaccharides. The comparison of ILSS with solid-phase synthesis, soluble-polymer-supported synthesis, and fluorous phase synthesis has been highlighted where applicable.
The THβC skeleton is found in numerous pharmacologically interesting compounds and hence these alkaloids have been in the focus of synthetic efforts for a long time. The most popular synthetic routes utilize the Pictet-Spengler cyclization, extensively reviewed in 1995 and more recently that could be considered as a biomimetic approach [22-24]. Alternatively, a rather similar Bischler-Napieralski cyclization [25] can be used. In a Bischler-Napieralski reaction, a tryptamide 6 is cyclized. Usually dehydration reagents, such as PCl5, POCl3, SOCl2 or ZnCl2, are needed to promote the loss of the carbonyl oxygen. The product of the Bischler-Napieralski reaction is a DHβC 7 which can then be further reduced to form the corresponding THβC (Figure 4).
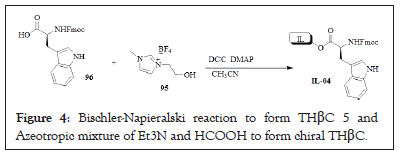
Figure 4: Bischler-Napieralski reaction to form THβC 5 and Azeotropic mixture of Et3N and HCOOH to form chiral THβC.
Chirality can be introduced to the DHβC product by using asymmetric reduction protocols. Asymmetric Transfer Hydrogenation (ATH) using Noyori-type catalysts offers a powerful method of accessing a chiral THβC skeleton. Due to the highly stereoselective nature of the reaction in question, this remains one of the most commonly employed procedures. Classical Noyori conditions use an azeotropic mixture of Et3N and HCOOH as the hydrogen source to reduce compound to the corresponding chiral THβC (Figure 4).
In addition to Aluminium Trihydrate (ATH), the stereochemistry of the reduction product can be controlled also by preexisting directing moieties in a diastereoselective fashion. In Woodward’s classic total synthesis of reserpine, published in 1958, a Bischler-Napieralski reaction from amide 10 to DHβC 11 was followed by a NaBH4 reduction selectively forming THβC 12. Interestingly but not very surprisingly, this reduction selectively yielded the wrong diastereomer [26]. However, in this case the configuration at C1 could be inverted at a later stage of the synthesis.
The stereochemistry in the THβCs can also be controlled by using chiral inductors in the Pictet-Spengler reaction. Internal induction as a means to control the stereochemistry at C1 uses chiral starting materials that are often derived from tryptophan. The existing stereochemistry guides the formation of the second chiral center in cases when C1 is substituted [27,28]. The diastereoselectivity of Pictet-Spengler reaction has been studied and discussed in detail [29]. The conformation of the spiroindolenine intermediate determines whether a trans-or a cis-product is formed (Figure 5). The trans-product is predominantly formed under thermodynamic control and under kinetic control the selectivity is turned towards the cis-product. However, the overall control of the cis/trans-selectivity is very complicated; in addition to the reaction temperature, the substitution pattern together with the size and electronic properties of the substituents have a considerable impact on the selectivity.
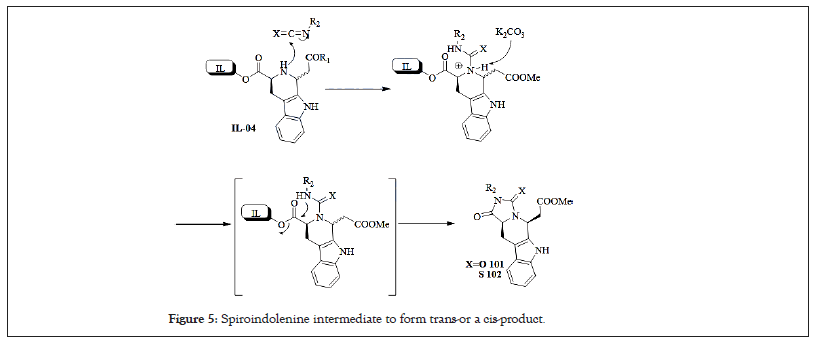
Figure 5: Spiroindolenine intermediate to form trans-or a cis-product.
Despite the complicated nature of this type of internal chiral induction, the reaction outcome has the potential of being highly stereoselective. It has been used extensively in indole alkaloid synthesis to control the stereochemistry at C1 [30]. An early example of successful use of internal induction is found in the ajmaline synthesis by Cook (Figure 6) [31]. In this work, tryptophan benzyl ester 13 was used for the Pictet-Spengler reaction. The yield of the trans-product was enhanced by acid induced epimerization that was conducted simultaneously with the Pictet-Spengler reaction.
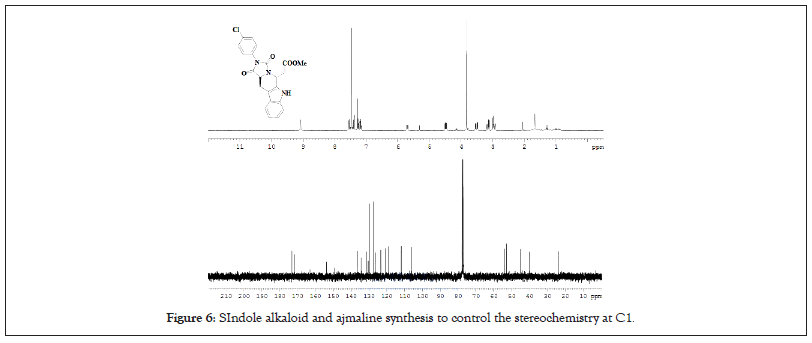
Figure 6: SIndole alkaloid and ajmaline synthesis to control the stereochemistry at C1.
The key in the epimerization is a reversible ring opening that favors the thermodynamically more stable trans-product (Figure 7). Hence, a reliable protocol exists to yield trans-product in very high selectivity from N2 benzyl substituted tryptophan derivatives. The same strategy to reach intermediate 15 has been successfully used to synthetize other related alkaloids such as 11-methoxymacroline and alstophylline [32].
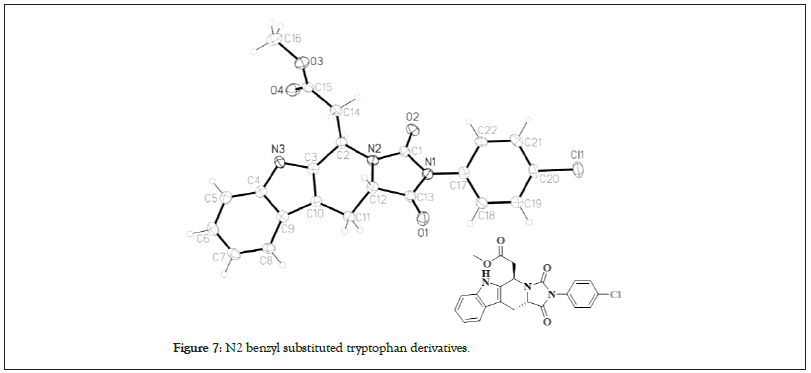
Figure 7: N2 benzyl substituted tryptophan derivatives.
Pictet-Spengler reactions and found that in addition to trans-selectivity, under suitable reaction conditions and substitution pattern, the Pictet-Spengler reaction can become highly cis-selective. In a representative example (Figure 8), the cyano substituent in the tryptophan derivative 16 is necessary for the reaction outcome to achieve good cis-selectivity, to form product. The kinetically controlled reaction has been subsequently used eg: In (-)-raumacline synthesis and the conditions leading to the cis-selectivity have been studied thereafter [33-35].
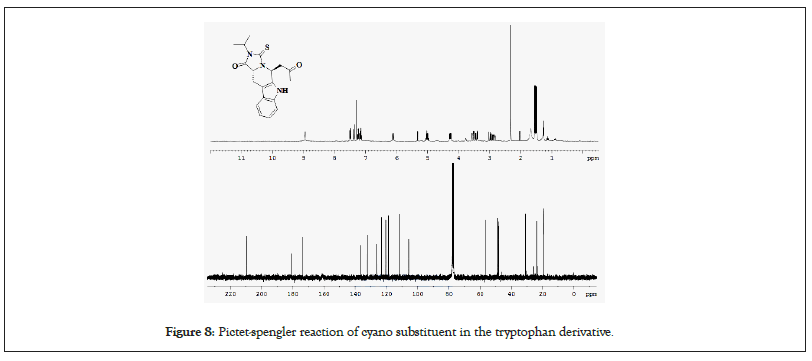
Figure 8: Pictet-spengler reaction of cyano substituent in the tryptophan derivative.
In addition to a directing group at C3, also chiral auxiliaries on N2 have been studied as an alternative. A benefit of an auxiliary on the nitrogen would be the easy attachment and removal of the chiral auxiliary. However, simple benzyl-or naphthyl-derived chiral groups provide only moderate diastereoselectivity and only 30%-80% [36]. Yet, good diastereoselectivities have been obtained using N,N-phthaloylamino acids (Figure 9) [37]. In this example the pre-formed imine 18 is protected with a phthaloylamino acid derivative and the N-protected THβC 19 is formed diastereoselectively.
Figure 9: Benzyl-or naphthyl-derived chiral groups to moderate diastereoselectivity to obtain N,N-phthaloylamino acids.
Moreover, the source of stereochemical information in Pictet-Spengler reactions can be from chiral carbonyl compounds. Condensed tryptamine 4 with a chiral aldehyde 20 derived from L-glutamic acid (Figure 10) [38]. The preferred cis-compound was formed exclusively when a Carboxybenzyl (Cbz) protecting group was used (R=Cbz) and the selectivity was turned towards the trans-product when the amine was protected with a pyrrole and speculated that the size of the protecting group is an important factor, but since pyrrole and Cbz-protecting groups are rather similar in size it seems more likely that this selectivity is guided by other factors. External asymmetric induction can also be used in the Pictet-Spengler reaction. The first enantioselective Pictet-Spengler reactions using external asymmetric induction were conducted in 1996. using diisopinocampheylchloroboranes and reaching 90% ee (enantiomeric excess) [39]. Today, various asymmetric reagents have been used for Pictet-Spengler reactions providing moderate to high ee:s. In recent publications, popular catalysts in asymmetric Pictet-Spengler reactions includes thiourea based catalysts [40,41] and chiral phosphoric acid diesters [42,43] (Figure 9).
Figure 10: Condensed tryptamine 4 with a chiral aldehyde 20 derived from L-glutamic acid.
Several THβCs have been reported to exhibit antiprotozoal, most notably antimalarial, activity. Malaria is one of the most important infectious diseases in the world. According to the World Health Organization (WHO) 200-300 million people are infected and 1.5-2.5 million people die of malaria annually. Some 90% of malaria deaths occur in Africa and 85% of the deceased are younger than 5 years-old [44-49]. Malaria is caused by red blood cell infected by protozoan parasites belonging to the Plasmodium genus, mainly Plasmodium falciparum [50]. Traditionally, malaria has been treated with quinine type drugs such as chloroquine. However, the emergence of drug resistant strains has created new challenges for efficient treatments [51]. Several recent studies have focused on the use of different THβC type compounds in the treatment of malaria.
(+)-7-Bromotrypargine is a marine natural product that was recently isolated from a sponge, Ancorina species and reported the isolation and the structural elucidation of the compound together with tests towards antimalarial activity. The compound was tested against both chloroquine-resistant (Dd2) and chloroquine-sensitive (3D7) strains of P. falciparum and (+)-7-bromotrypargine was shown to display IC50 values of 5.4 μM (Dd2) and 3.5 μM (3D7). Similar compounds were also studied and moderate antimalarial activity was reported.
In 2012, synthesized a series of simple 1-substituted THβC derivatives with the general structure with one or more substituents on the phenyl moiety. They tested a series of 20 compounds against the W2 culture adapted strain of P. falciparum resistant to chloroquine, pyrimethamine and proguanil and nine compounds showed antiplasmodial activity. The most active compound was a para-methoxy-substituted one with IC50 of 0.7 µM (W2 IC50 of chloroquine 0.7 µM).
In 2008, synthesized a series of chloroquine-THβC hybrid molecules with the general structure. Altogether 23 compounds were screened against chloroquine sensitive P. falciparum strain and the most active compounds had R=i-Pr, R=Me and R=Et and showed Minimum Inhibitory Concentrations (MIC) of 0.05, 0.06, and 0.11 μM, respectively, thus showing significantly greater activity than the standard drug chloroquine (MIC=0.391 μM).
A new class of potent antimalarials that has recently gained attention is spiroindolones with a THβC structure. In 2010, these types of compounds were recognized as antimalarials in high-throughput screenings by the Novartis Institute of Tropical Diseases. These compounds act against P. falciparum with a mechanism distinct from that of the existing antimalarial drugs and the optimized lead compound NITD609 has a very high activity of IC50=0.2 nM. In 2012, NITD609 entered phase 2 clinical trials.
In addition to antimalarial studies, THβCs have recently also gained attention as potential anti-leishmanial and trypanocidal compounds. Leishmaniasis is a tropical infectious disease and the number of people infected with leishmaniasis is ~ 12 million. The annual incidence of leishmaniasis is ~ 2 million cases and the numbers are increasing. Leishmaniasis is caused by the protozoan flagellate Leishmania species, most notably L. donovani, which is spread by sand flies (Phlebotomus and Lutzomyia species). About 90% of leishmaniasis cases occur on the Indian Peninsula, in Brazil and in Sudan.
Trypanosoma species cause trypanosomiasis that can either be manifested as African trypanosomiasis (sleeping sickness) caused by T. brucei or as Chagas disease caused by T. cruzi. The incidence of African trypanosomiasis is 50,000-70,000 cases annually and it is endemic to the tropical Africa, while Chagas disease occurs in the Middle and Southern America. The approximated number of people with Chagas disease is 8-11 million.
It has been known for a long time that such complicated THβC alkaloids as α -yohimbine, corynanthine and buchtienine exhibit antileishmanial activity [52,53]. However, during the last 5 years a new interest has arisen towards smaller, synthetic THβC derivatives and several publications have reported antileishmanial activity. In 2010, synthesized a series of indolylglyoxylamides with the general structure and reported good antileishmanial activities with IC50 values of 3.79 µM and 5.17 μM for the ortho-bromosubstituted and para-ethylated compounds, respectively [54]. These values were several folds better than the standard drug activities (IC50 of pentamidine: 20.43 μM) and reported triazine derivatives as well as other similar derivatives 35 as leishmanicidals [55,56]. The triazino derivatives have also been tested in vivo of antimalarial compounds with the general structure for antileishmanial activity. A para-bromosubstituted compound showed the most promising inhibitory activity towards L. donovani, with IC50 value of 6.1 μM (IC50 of pentamidine: 6.3 μM).
Some THβC derivatives have also been studied for trypanocidal activity. In 2010, Tonin and Valdez published studies on similar THβC derivatives [57]. These compounds showed promising activity and compound has been further studied for synergistic activity with other medication but these publications remain the only publications so far on trypanocidal activity of THβC derivatives [58].
After establishing that alkynes can undergo Pictet-Spengler reactions on supports the reaction previously developed in the laboratory can be subsequently derivatized to achieve the maximum functional group of the drug. According to the requirements of optimization, the ester group on the unsaturated alkyne can also be used for subsequent ring closure to generate more rigid heterocyclic molecules. The general strategy of phylogenetically profiling degenerate Polymerase Chain Reaction (PMC) derived amplicons to guide gene cluster selection and transcription factor overexpression to activate recovered gene clusters of interest can easily be adapted to the discovery of new members of other previously rare families of bioactive natural products from large metagenomic libraries. As traditional natural product discovery methods saturate the chemical space of frequently occurring bacterial secondary metabolites, homology-guided metagenomic library screening should be useful for the routine discovery of novel natural products that expand on previously underexplored bioactive natural product families.
This work was supported by National Science Council Taiwan; National Institutes of Health Grant GM077516. S.F.B. is a Howard Hughes Medical Institute Early Career Scientist.
Citation: Thummanagoti S (2023) Synthesis and Investigation of Ionic Liquid Supported Tetrahydro-β-carboline Derivatives as Inhibitors of the Cancer Resistance Protein (ABCG2) Indolotryptoline Anti-Proliferative Agents under Microwave Irradiation. J Clin Chem Lab Med. 6:265.
Received: 20-Feb-2023, Manuscript No. JCCLM-23-21833; Editor assigned: 22-Feb-2023, Pre QC No. JCCLM-23-21833 (PQ); Reviewed: 08-Mar-2023, QC No. JCCLM-23-21833; Revised: 15-Mar-2023, Manuscript No. JCCLM-23-21833 (R); Published: 22-Mar-2023 , DOI: 10.35248/JCCLM.23.6.265
Copyright: © 2023 Thummanagoti S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.