Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2017) Volume 6, Issue 1
In the presence of trifluoracetic acid on the basis of three-component condensation of thiourea with its different aldeyhde (benzaldehyde, p-tolylaldehyde and acetaldehyde) and β-diketones (diethyl malonate, acetylacetate, ethyl acetoacetate, ethyl 2-ethylacetoacetate, 2-ethyl acetoacetate) an efficient method for the synthesis of tetra(hexa) hydropyrimidinethione- carboxylates has been worked out.
Keywords: Three-component condensation; Thiourea; Diethyl malonate; Tetra(hexa)hydro-pyrimidines
The need for the new researches for raising the efficiency of in the development of the petrochemical and medical industry is noticeable. Especially, there is a specific fundamental and applicable issue in science that the approach on a scientific direction in their solution does not justify itself and is not achieved satisfactory results for many years. The need for a complex scientific approach arises for such issues and the most successful scientific results of recent years are found in the research of the intersection of science with the multidisciplinary approach. From this point of view, as a result of the most recent studies conducted in the Institute of Chemistry of Additives of the Azerbaijan National Academy of Sciences, it was determined that, biocide additives synthesized at the institute shows biological (physiological) activity in other areas too, in addition to protect the oil products from the microbiological damage. For instance, amino ethanol and some derivatives of amino alcohols synthesized by the employees of Institute shows the properties of anesthetic and terminal anesthesia for the mucous membranes of the eye. The studies were conducted at the first Moscow State Medical Institute named after Sechenov IM. These substances are 7 times greater compared to the widely-used “xycain”, according to its activity. As a result of investigations carried out, in the “Biochemical Pharmacology” Laboratory (Moscow oblast, Kupavna settlement) of Scientific Research Institute for the “Biological Testing” of chemical compounds, their antialcohol properties have been also identified.
Bacteriological influence of heteroaryl sulfamides containing pyrimidine and pyrazole was investigated in the Microbiology department of Baku State University, and it was determined that, these substances quickly perishes the “stafilococ”, “penicillium” and “mold” mushrooms in the concentration of 0,05-0,1%. 3 (beta-hydroxy (oxymethylene)-ethyl-1,3-oxazolidine (was named as “Cyclazole”) has a high disinfectant propertiesand its usage was allowed by the Ministry of Health of the Republic of Azerbaijan. In other words, as a result of tests conducted at the Institute for Scientific Research of Virology, Microbiology, it was determined that the nitrogenous derivatives of alkylphenol and alkylaromatic ketones have the highest disinfectant and antiseptic properties and was advised to apply.
When it comes to the use of these compounds in the oil and chemical industry, it should be noted that, many industrial materials, including the oil products are damaged with microorganisms when they are maintained and their exploitation conditions are violated (the highest humidity and temperature). Microorganisms affects negatively to the composition, properties and quality of these damaged materials, by adopting the hydrocarbons of petroleum products, as a source of food and energy and as well as affecting them by the metabolic products. Because of the fact that, the problem of microbiological damage of industrial purpose and other materials is in large scale and the amount of the economic loss is too great, it is in the focus of many scientists currently [1-4].
More effective method of fighting against the microbiological loss is the use of antimicrobial properties of the compounds. The experience of foreign and countrywide researchers and users has shown that, especially these substances provide reliable and long-term protection of industrial materials.
“Biobor JF”, which is processed by the companies New York Central Reilrood and Standart Oil Co for the protection and preservation of Jet and aircraft fuels. 4-octyl isothiazolone (firm name of biocide Kathon LP) and 2-octyl-4-isothiazolin-3-on (СКАН М-8) [5] is used for the preservation of liquid fuels. Vazin [6], Grotan UK [7], Florachit [8] biocides are applied for the protection of cutting fluids. These biocides are effective against the bacteria.
It’s more than 40 years that the scientific studies are carried out directed to the research of bio-sustainability of oil products and their protection through the biocides. For this purpose, purposeful synthesis of chemical compounds having high biological active properties is carried out [9]. The biological activity of synthesized compounds in oil products (oil, fuel and CF) was studied and the mutual relationship between their chemical structure and antimicrobial activities was determined.
From this point of view in the present case, expansion of scientific research on synthesis of new heterocyclic compounds containing multifunctional nitrogen and sulfur and their application as an antimicrobial and antioxidant was designed [10,11].
New cyclic thiureas were obtained by continuing researches in the field of the synthesis of various classes of organic sulfur compounds and the study of their transformations.
So that, ethyl 6-ethoxy-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine- 5-carboxylate (1), 1-(6-methyl-2-thioxo-4-(p-tolyl)- 1,2,3,4-tetrahydropyrimidin-5-yl)ethanone (2), ethyl 6-methyl-4-phenyl- 2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (3), ethyl 4,5-dimethyl-2-thioxo-6-(p-tolyl)hexahydropyrimidine-5-carboxylate (4), 1-(5-ethyl-4,6-dimethyl-2-thioxohexa-hydropyrimidin-5-yl) ethanone (5) were obtained for the first time by us based on the trifluoro- acetic acid catalyst.
Chemistry
Synthesis of ethyl 6-ethoxy-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine- 5-carboxylate (1): Thiourea (1.52 gr, 0.02 mol) is dissolved in the ratio of 5:1 of acetylacetone (5 ml) and ethyl alcohol (1 ml) and diethyl malonate (3.04 ml, 0.02 mol) is added on it by drops. After being dissolved in the magnetic stirrer for 5 minutes, benzaldehyde (2.03 ml, 0.02 mol) is added on it. After being dissolved in the stirrer for 30 minutes, 0.02 ml Trifluoroacetic acid catalyst is added on it and mixed by heating at 60-65°C. The progress of the reaction is controlled by Sulifol UV 254 plate. After determining the full completion of reaction, solution is evaporated and is cleansed in ethyl alcohol solution. The white crystalline having the melting temperature of 210°C is obtained. The yield is 2.1 g.
Synthesis of 1-(6-methyl-2-thioxo-4-(p-tolyl)-1,2,3,4-tetrahydropyrimidin- 5-yl)ethanone (2): Thiourea (0.76 gr, 0.01 mol) is dissolved in the ratio of 4:1 of acetylacetone (4 ml) and ethyl alcohol (1 ml) and acetylacetate (0.69 ml, 0.01 mol) is added on it by drops. After being dissolved in the magnetic stirrer for 5 minutes, p-tolylaldehyde (1.18 ml, 0.01 mol) is added on it. After being dissolved in the stirrer for 30 minutes, 0.03 ml trifluoroacetic acid catalyst is added on it and mixed by heating at 55-60°C. The progress of the reaction is controlled by Sulifol UV 254 plate. After determining the full completion of reaction, solution is evaporated and is cleansed in ethyl alcohol solution. The white crystalline having the melting temperature of 215°C is obtained. The yield is 1.5 g.
Synthesis of ethyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4- tetrahydropyrimidine-5-carboxylate (3): Thiourea (0.51 gr, 0.0067 mol) is dissolved in the ratio of 6:1 of acetylacetone (6 ml) and ethyl alcohol (1 ml) and ethyl acetoacetate (0.84 ml, 0.0067 mol) is added on it by drops.
After being dissolved in the magnetic stirrer for 5 minutes, benzaldehyde (0.68 ml, 0.0067 mol) is added on it. After being dissolved in the stirrer for 30 minutes, 0.03 ml trifluoroacetic acid catalyst is added on it and mixed by heating at 65-70°C. The progress of the reaction is controlled by Sulifol UV 254 plate. After determining the full completion of reaction, solution is evaporated and is cleansed in ethyl alcohol solution. The white crystalline having the melting temperature of 152°C is obtained. The yield is 1.2 g.
Synthesis of ethyl 4,5-dimethyl-2-thioxo-6-(p-tolyl)hexahydropyrimidine- 5-carboxylate (4): Thiourea (1.52 gr, 0,02 mol) is dissolved in the ratio of 6:1 of acetylacetone (6 ml) and ethyl alcohol (1 ml) and ethyl 2-ethylacetoacetate (3.23 ml, 0.02 mol) is added on it by drops.
After being dissolved in the magnetic stirrer for 25 minutes, p-tolylaldehyde (2.36 ml, 0.02 mol) is added on it. After being dissolved in the stirrer for 30 minutes, 0.02 ml trifluoroacetic acid catalyst is added on it and mixed by heating at 65-70°C. The progress of the reaction is controlled by Sulifol UV 254 plate. After determining the full completion of reaction, solution is evaporated and is cleansed in ethyl alcohol solution. The white crystalline having the melting temperature of 211°C is obtained. The yield is 2.31 g.
Synthesis of 1-(5-ethyl-4,6-dimethyl-2-thioxohexahydropyrimidin- 5-yl)ethanone (5): Thiourea (1.52 gr, 0.02 mol) is dissolved in the ratio of 3:1 of acetylacetone (3 ml) and ethyl alcohol (1 ml) and 2-ethyl acetoacetate (3.23 ml, 0.02 mol) is added on it by drops.
After being dissolved in the magnetic stirrer for 40 minutes, acetylaldehyde (1.12 ml, 0.02 mol) is added on it. After being dissolved in the stirrer for 30 minutes, 0.02 ml trifluoroacetic acid catalyst is added on it and mixed by heating at 70-75°C. The progress of the reaction is controlled by Sulifol UV 254 plate. After determining the full completion of reaction, solution is evaporated and is cleansed in ethyl alcohol solution. The white crystalline having the melting temperature of 209°C is obtained. The yield is 2.1 g (Scheme 1; Table 1).
| No. | Compounds | Mp, °? | Brutto formula |
Element analysis, Found/Calculated, % |
Yield, % | Spectrum analysis | |
|---|---|---|---|---|---|---|---|
| N | S | ||||||
| 1 | 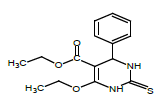 |
210 | C15H17N2O3S | 9.12/9.18 | 10.41/10.49 | 61 | 1H NMR (300 MHz, DMSO-d6): 2.79 (s., 3H, CH3); 3.22 (s., 3H, CH3); 5.12 (d., 1H, CH-Ar); 5.63 (s., 4H, 4CH-Ar); 7.41 (s., 1H, NH); 9.11 (s.,1H, NH). 13C NMR (75 MHz, DMSO-d6): 14.24, 18.33, 19.48, 39.95, 51.05, 59.99, 99.24, 126.92, 130.05, 139.40, 152.08, 166.27. |
| 2 | 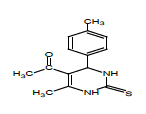 |
197 | C14H15N2OS | 10.77/10.81 | 12.29/12.36 | 58 | 1H NMR (300 MHz, DMSO-d6): 2.07 (s., 3H, CH3); 2.25 (s., 3H, CH3); 2.26 (s., 3H, CH3); 5.20 (d., 1H, CH-Ar); 7.12 (s., 4H, 4CH-Ar); 7.77 (s., 1H, NH); 9.15 (s.,1H, NH). 13C NMR (75 MHz, DMSO-d6): 19.33 (CH3); 21.11 (CH3); 30.69 (CH3); 54.01 (CH-Ar); 110.01 (-C=); 126.82 (2CH-Ar); 129.50 (2CH-Ar); 137.64 (-C=); 141.76 v? 148.26 (-C-); 152.58 (C=S); 194.78 (C=O). |
| 3 | 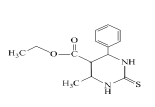 |
152°C | C14H15N2O2S | 10.12/10.18 | 11.59/11.63 | 65 | 1H NMR (300 MHz, DMSO-d6): 2.09 (t., 3H, CH3); 2.75 (s., 3H, CH3); 2.92 (s., 3H, CH3); 3.67 (k., 2H, CH2O); 7.31 (d., 1H, NH); 7.35 (d., 2H, NH); 8.74 (s., 1H, NH); 8.89 (s., 1H, NH). 13C NMR (75 MHz, DMSO-d6): 19.31 (CH3); 55.41 (CH-Ar); 57.65 (CH2O); 126.75 (2CH-Ar); 128.85 (2CH-Ar); 155.60 (C=S); 167.80 (-O-C=O). |
| 4 | 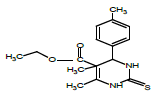 |
211 | C16H20N2O2S | 9.12/9.21 | 10.47/10.53 | 54 | 1H NMR (300 MHz, DMSO-d6): 2.79 (s., 3H, CH3); 2.82 (s., 3H, CH3); 4.39 (d., 4H, 2CH2O); 5.22 (d., 1H, CH-Ar); 5.83-5.91 (s-s., 2H, =CH2); 7.21 (m., 5H, 5CH-Ar); 7.55 (s., 1H, NH); 8.99 (s., 1H, NH). 13C NMR (75 MHz, DMSO-d6): 24, 29, 39, 51, 83, 118, 122, 124, 128, 133, 142, 150, 180 (C=S), 204 (C=O). |
| 5 | 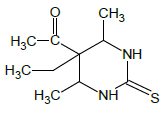 |
209 | C10H16N2OS | 13.17/13.21 | 15.02/15.09 | 65 | 1H NMR (300 MHz, (DMSO-d6, d): 2.19 (s., 3H, CH3); 2.23 (s., 3H, CH3); 3.26 (s., 3H, CH3); 4.22 (d., 1H, CH-Ar); 5.32 (s., 4H, 4CH-Ar); 7.76 (s., 1H, NH); 9.17 (s., 1H, NH). 13C NMR (75 MHz, DMSO-d6): 14.99, 25.89, 62.35, 86.28, 116.85, 122.55, 125.01, 126.21, 126.22, 128.87, 128.88, 130.01, 133.24, 142.16, 151.96, 167.91, 179.05. |
Table 1: Physical and chemical characteristics of some cyclic thioureas.
In the presence of trifluoracetic acid new effective synthesis method of tetra(hexa)hydropyrimidinethione-carboxylates (1-5) have been worked out. It was determined that in the presence of trifluoracetic acid from mutual influence of aldehydes with β-diketones and thiourea in the alcohol environment with 50-75% outputs the relevant different new compounds are obtained. The three-component condensation reactions come to an end within 2,5-3 hours at 60-75°C. Synthesis has been carried out on the following (Scheme 2).
Scheme 2: Initially, the protonated imine is formed by a combination of proton. This cation reacts with enol form of ketoester and forms thoureide. In last stage cyclization occurs with the separation of water molecule. As seen from reaction mechanism enol form of ß-diketoether is involved in the reaction. Keto-enol tautomerism is observed as a result of increased C-H acidity since methylene group in a-condition is associated with electron acceptor carbethoxy compared to carbonyl group in ß-diketoether. When acetoacetic ether passes to enol form, energy absorption arising from formation of intramolecular hydrogen bond or connected double bonds system simplifies enolization and stabilizes enol form.