Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Research Article - (2013) Volume 6, Issue 7
Ten novel transition metal complexes of N,O donor ligands have been synthesized by the reaction of ligands with metal acetates in 2:1 molar ratio. These reactions were carried out under reflux in methanol for 3-6 hours. The ligands and their transition metal complexes were characterized on the basis of physical properties, elemental analysis, Infrared spectroscopy, Magnetic susceptibility, Ultraviolet and Atomic absorption spectroscopy and 1H-13C NMR spectroscopy. The FT- IR spectra of the complexes indicated that ligands behaved in bidentate manner coordinating via the nitrogen and oxygen. 1H-NMR and 13C-NMR data of the ligands in pure and complex form confirmed the formation of the complexes. Other analytical techniques demonstrated that transition metal complexes of Cu, Ni, Co and Zn are four coordinated and those of Mn are six coordinated. Furthermore, antibacterial activities have been discussed.
Keywords: Transition metal complexes; NO donor schiff bases; Spectral characterization and biological activity
The preparation and study of inorganic compounds containing biologically important ligands is made easier, because certain metal ions are active in many biological processes; species of low molecular weight are, hence, sought that reproduce, as far as possible, the structural properties and the reactivity of naturally occurring complexes of these ions in such processes. The fact that copper together with magnesium, calcium, iron, zinc, chromium, vanadium, and manganese are essential metallic elements and display great biological activity when associated with certain metal–protein complexes, participating in oxygen transport, electronic transfer reactions, or the storage of ions [1] has produced enormous curiosity in the study of systems containing these metals [2].
Schiff bases have a vital position in metal coordination chemistry even almost a century since their discovery. Due to their simplicity in preparation, diverse properties, medicinal, biochemical and industrial applications, the keen interest in the study of these compounds arose in the recent years. A number of metal coordination complexes of Schiff bases have been suggested as antibacterial, antifungal, cytotoxic, anti-inflammatory and Cytostatic agents. [3-6] In order to widen the scope of investigations on the coordination behavior of various donor ligands including Schiff base towards organo metallics, we carried out the investigations and established their bioactivities.[3,4,7-10] As an extension of this research, herein, we report the synthesis, characterization and bactericidal activity of some Schiff base metal complexes.
All the chemical reagents used were of analytical grade. 2,3-Dihydroxybenzaldehyde, 3-Chloroaniline, 3-Trifluromethylaniline and metal salts were obtained from Aldrich (U.S.A) chemicals and E.Merck (Germany). All the solvents which were used were of spectroscopic grade and were obtained from E.Merck (Germany) and Fluka Ltd. Melting points were determined in a capillary tube using electrothemal melting point apparatus Mitamura Riken Kogyo (Japan) model MPD. Infrared spectra were measured as KBr pellets on a FTIR spectrometer Bio-Rad Excalibur (USA) model FTs 300 MX in a frequency range of 4000-400 cm-1. The electronic absorption spectra of the ligands and complexes were recorded using DMSO as solvent on a UV-visible Spectrophotometer M/s Shimadzu 1600. The elemental analysis was performed on an Elemental analyzer LECO Corporation (USA) CHNS-932. 1H- NMR and 13C-NMR spectrum of ligands and their metal complexes were recorded using a FT-NMR spectrometer Bruker (Germany) 300 MHz.
Synthesis of ligands
The ligands were synthesized by the reported methods [11].
General procedure for synthesis of transition metal complexes
Transition metal complexes were prepared by mixing the corresponding ligands with transition metals Cu(CH3COO)2.2H2O, Co(CH3COO)2. 4H2O, Ni(CH3COO)2.4H2O, Mn (CH3COO)2.4H2O and Zn (CH3COO)2.2H2O.
Synthesis
To a solution of HL1 or HL2 in methanol (20 ml) a solution of metal salts in 15 ml methanol in 2:1 molar ratio was added drop wise. The reaction mixture was refluxed for 3-6 hours at 60°C. The precipitates were formed. The product was filtered and washed with commercial methanol. The resulting product was air dried. Solids products obtained were recrystallized in suitable solvents.
The reactions of Schiff bases with transition metal salts were quite facile and were completed in 3 to 8 hours, the water form during the reaction as byproduct was removed using Dean Stark funnel (Scheme 1 and 2).
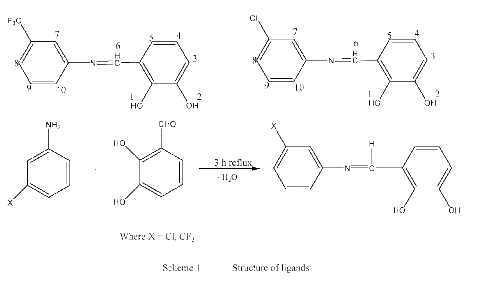
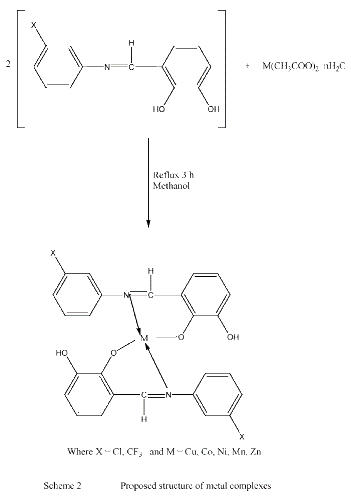
The complexes are air-stable, hygroscopic, with higher melting points, soluble in organic solvents. The results of the elemental analysis and some physical characteristics of the obtained compounds are quite satisfactory (Table 1).
| Comp. No | Empirical formula (formula weight) |
M.P. ºC |
Yield (%) | Colour | %C Calc. (Found) |
%H Calc. (Found) |
%N Calc. (Found) | %M Calc. (Found) |
|---|---|---|---|---|---|---|---|---|
| 1 | C14H10NO2F3 (281) | 134 | 87 | Orange | 57.78 (58.88) |
2.95 (3.03) |
4.48 (4.59) |
___ |
| 2 | C28H18N2O4F6.Cu(623) | >300 | 78 | Green | 53.51 (53.88) |
2.75 (2.98) |
4.38 (4.49) |
9.77 (10.02) |
| 3 | C28H18N2O4F6.Co(618) | >310 | 81 | Dark brown | 53.30 (54.30) |
2.35 (2.93) |
4.27 (4.54) |
8.91 (9.14) |
| 4 | C28H18N2O4F6. Ni(618) | >300 | 76 | Dark brown | 53.83 (54.20) |
2.32 (2.90) |
4.44 (4.52) |
8.86 (9.03) |
| 5 | C28H18N2O4F6.Zn(625) | >320 | 65 | Dark Mahron | 52.91 (53.72) |
2.77 (2.87) |
4.23 (4.47) |
8.67 (9.96) |
| 6 | C28H18N2O4F6Mn.2H2O(653) | >300 | 70 | Light Mahron |
51.42 (51.62) |
3.23 (3.37) |
4.08 (4.30) |
7.56 (7.99) |
| 7 | C13H10ClNO2(247) | 145 | 85 | Pinkish red | 63.03 (62.56) |
3.82 (3.91) |
4.90 (5.03) |
__ |
| 8 | C26H18N2O2Cl 2.Cu(556) | >300 | 78 | Brown | 55.06 (55.66) |
2.93 (3.04) |
4.83 (4.94) |
10.80 (11.02) |
| 9 | C26H18N2O2Cl 2 .Co(551) | >310 | 81 | Dark red | 53.30 (54.30) |
2.35 (2.93) |
4.27 (4.54) |
10.67 (10.88) |
| 10 | C26H18N2O2Cl 2. Ni(551) | >300 | 76 | Reddish | 54.57 (55.03) |
3.29 (3.44) |
4.55 (4.60) |
9.63 (10.03) |
| 11 | C26H18N2O2Cl 2. Zn(558) | >330 | 65 | Red | 56.05 (56.39) |
3.04 (3.13) |
4.86 (4.93) |
10.70 (10.96) |
| 12 | C26H18N2O2Cl 2.Mn.2H2O(587) | >330 | 70 | Brick red | 52.42 (52.62) |
3.29 (3.37) |
4.22 (4.30) |
9.40 (9.89) |
*In all other tables the formulation and number of the compounds are the same as given in this table.
Table 1: Physical data of the ligands and their metal complexes
Infrared spectroscopy
The common region for infrared analysis in organo transition chemistry is 4000-200 cm-1. IR spectra of all the compounds have been recorded using KBr pellets in the range 4000-400 cm-1. The characteristic absorption bands are listed in Table 2. The important absorption frequencies are ν (OH),ν(M-O), ν(M-N) and ν(-C=N).
| Codes | n O-H | n C=N | n M-O | n M-N |
| (1) | 3316 | 1619 | - | - |
| (2) | 3404 | 1604 | 430 | 539 |
| (3) | 3430 | 1591 | 415 | 518 |
| (4) | 3455 | 1596 | 434 | 535 |
| (5) | 3435 | 1601 | 413 | 536 |
| (6) | 3440 | 1589 | 469 | 531 |
| (7) | 3439 | 1621 | - | - |
| (8) | 3343 | 1602 | 417 | 544 |
| (9) | 3440 | 1601 | 414 | 528 |
| (10) | 3415 | 1596 | 418 | 534 |
| (11) | 3428 | 1584 | 416 | 533 |
| (12) | 3413 | 1585 | 416 | 534 |
Table 2: Infrared spectra of the ligands and metal complexes.
Assignment of different vibration bands have been made by the comparison of ligand spectra with that of transition metal complexes. In the spectra of the ligands a strong band at 3300 and 3450 cm-1 was assigned to –OH stretching vibration. After complex formation this band was still observed in case of Mn complexes only, supporting the fact that water molecule is present as water of coordination.
In the low frequency region, the band observed in the complexes in the region of 500-550 cm-1 and 400-470cm-1 is attributed to (M-N) and ν(M-O) respectively [7]. The IR data suggested that the metal was bonded to the Schiff base (Table 2).
UV-Visible spectroscopy
UV-Visible studies were made in DMSO. From the values of λmax obtained it was found that n→ π* and π→ π* transitions are observed which are due to chromophoric groups (imine and aromatic ring) in the ligands. The spectra of the complexes showed red shift. All solutions were of 10-4 molar concentration; due to this dilute concentration no d→d transitions were observed [13]. The obtained values were compared to literature values and it was found that values for copper, nickel and cobalt were close to the values for four coordinated systems and for manganese the values were in the range of six coordinated system (Table 3).
| Compound | Wavelength (λ) | Absorption (A) | Type of transitions |
|---|---|---|---|
| (1) | 354, 230 | 1.50, 0.50 | n-p * and p- p * |
| (2) | 440,234 | 1.67, 0.55 | n-p * and p- p * |
| (3) | 477,236 | 1.75, 0.751 | n-p * and p- p * |
| (4) | 412,265 | 1.65, 0.67 | n-p * and p- p * |
| (5) | 383,245 | 1.57, 0.852 | n-p * and p- p * |
| (6) | 404,247 | 1.64, 0.573 | n-p * and p- p * |
| (7) | 365,276 | 1.571,0.541 | n-p * and p- p * |
| (8) | 450,260 | 1.75, 0.511 | n-p * and p- p * |
| (9) | 414,287 | 1.62, 0.43 | n-p * and p- p * |
| (10) | 386,290 | 1.58, 0.47 | n-p * and p- p * |
| (11) | 411,276 | 1.60, 0.852 | n-p * and p- p * |
| (12) | 432,268 | 1.73, 0.573 | n-p * and p- p * |
Table 3: UV spectral data of ligands and its Synthesized Transition Metal Complexes.
Magnetic susceptibility
Magnetic susceptibility is quite useful technique for determining unpaired electrons in paramagnetic complexes which in turns help in suggesting structure of the complexes and the nature of ligand. Ligands were strong field ligands and this fact was supported by magnetic moment values for metals. The values indicated that ligand was strong field ligand as all electrons in nickel have been paired up. Value of magnetic moment for nickel is 0.72 B.M which indicated that there was no unpaired electron left after complex formation. Value should be higher if the electrons were unpaired. For other metals like copper, cobalt and manganese the values were in the range of 1.59-1.72 which was close to literature value for one unpaired electron [14]. So it was taken as evidence that all metals were in +2 oxidation state (Table 4).
| Codes | μ (B.M) (Found) |
μ (B.M) (Literature values) |
Unpaired electrons |
|---|---|---|---|
| (2) | 1.60 | 1.73 | 1 |
| (3) | 1.67 | 1.73 | 1 |
| (4) | 0.72 | - | 0 |
| (6) | 1.59 | 1.73 | 1 |
| (8) | 1.63 | 1.73 | 1 |
| (9) | 1.70 | 1.73 | 1 |
| (10) | 0.41 | - | 0 |
| (12) | 1.61 | 1.73 | 1 |
Table 4: Magnetic susceptibility of Synthesized Transition Metal Complexes.
Multinuclear NMR
H-NMR Data: 1H-NMR spectra of all compounds had been recorded on 300 MHz NMR spectrometer. The characteristic resonance peaks in the 1H NMR spectra of ligand were recorded in CDCl3 and its metal complexes in DMSO. The expected resonance was assigned by intensity pattern and integration. The integration of spectra showed good agreement with the composition of the compounds. In both ligand (HL1, HL2) hydroxyl proton showed signal at 13.23 ppm and 12.31 ppm respectively. These signals disappeared in all complexes due to the deprotonation of OH [15,16]. Both ligands HL1, HL2 showed a simple pattern for aromatic protons which is due to presence of only seven protons in this region. These protons do not show any significant change. As ligand to metal ratio was 2:1, therefore, signals for 14 protons were observed after complex formation. The azomethanic protons of the ligands gave signal at 8.63 and 8.86 for HL1 and HL2 respectively. The downfield shift of both the signals to 8.99 and 8.94 suggests the bonding of N to the metal (Table 5).
| H-NO | (1) | (5) | (7) | (11) |
| 1 | 13.23(s)[2H] | - | 12.31(s){2H] | - |
| 2 | 13.23(s)[2H] | 12.67(s)[2H] | 12.31(s){2H] | 12.75(s)[2H] |
| 3 | 6.87-7.58(m)[7H] | 6.90-7.78(m)[14H] | 6.60-7.7.80(m){7H] | 6.80-7.54(m)[14H] |
| 4 | 6.87-7.58(m)[7H] | 6.90-7.78(m)[14H] | 6.60-7.7.80(m){7H] | 6.80-7.54(m)[14H] |
| 5 | 6.87-7.58(m)[7H] | 6.90-7.78(m)[14H] | 6.60-7.7.80(m){7H] | 6.80-7.54(m)[14H] |
| 6 | 8.63(s)[1H] | 8.99(s)[2H] | 8.86(s)[1H] | 8.94(s)[2H] |
| 7 | 6.87-7.58(m)[7H] | 6.90-7.78(m)[14H] | 6.60-7.80(m)[7H] | 6.80-7.54(m)[14H] |
| 8 | 6.87-7.58(m)[7H] | 6.90-7.78(m)[14H] | 6.60-7.80(m)[7H] | 6.80-7.54(m)[14H] |
| 9 | 6.87-7.58(m)[7H] | 6.90-7.78(m)[14H] | 6.60-7.80(m)[7H] | 6.80-7.54(m)[14H] |
| 10 | 6.87-7.58(m)[7H] | 6.90-7.78(m)[14H] | 6.60-7.80(m)[7H] | 6.80-7.54(m)[14H] |
Table 5: 1H NMR spectral data of ligands and its Synthesized Transition Metal Complexes.
C NMR Data: The 13C NMR spectral data of ligand and its metal complexes were recorded in DMSO. The C1 attached to OH group shifted to a lower field region in complexes, indicating participation of the C-O group in coordination to transition metal to form C-O-M bonds. The resonances due to the aromatic C-atoms did not shift significantly on bonding to transition metals. The azomethinic C -7 showed a down field shift due to shifting of electron density from nitrogen to metal supporting the formation of N-M bond (Table 6).
| C.NO | (1) | (5) | (7) | (11) |
| C1 | 148.57 | 149.51 | 148.93 | 149.66 |
| C2 | 145.04 | 145.81 | 145.07 | 146.10 |
| C3 | 118.43 | 118.43 | 118.23 | 119.39 |
| C4 | 123.40 | 123.41 | 121.25 | 121.39 |
| C5 | 123.50 | 123.55 | 123.29 | 123.38 |
| C6 | 119.51 | 119.85 | 118.39 | 119.80 |
| C7 | 164.00 | 166.21 | 163.45 | 164.72 |
| C8 | 148.73 | 149.83 | 149.17 | 150.85 |
| C9 | 121.99 | 122.66 | 119.63 | 120.17 |
| C10 | 132.20 | 131.35 | 135.15 | 134.34 |
| C11 | 123.55 | 123.40 | 127.02 | 126.96 |
| C12 | 131.33 | 131.35 | 130.52 | 130.80 |
| C13 | 130.11 | 130.92 | 119.38 | 119.59 |
| C14 | 123.86 | 123.92 | - | - |
Table 6: 13C NMR spectral data of ligands and its Synthesized Transition Metal Complexes.
Biological activity
The antibacterial activity of Schiff base and metal complexes against Staphylococcus aureus strain was screened using agar well diffusion method [17]. Imipenum was used as standard drug. The inhibition zones were measured in mm. The zone of inhibition of std. drug was 26 mm. The size of well was 6 mm (diameter) std. and wells were supplemented with DMSO and reference antibacterial drugs. The concentration of test sample was 1 mg/ml of DMSO. The plates were incubated at 37ºC for 24 hrs. The result indicated that both ligands showed significant activity. Whereas, Mn complex of HL1 and Ni complex of HL2 showed significant activity against standard drug (Table 7).
| Compound Codes | Zone of Inhibition (mm) |
Zone of inhibition of Std. drug, mm |
|---|---|---|
| 1 | 22.87 | 26 |
| 2 | 10.13 | 26 |
| 4 | 11.01 | 26 |
| 6 | 16.55 | 26 |
| 7 | 17.04 | 26 |
| 10 | 20.54 | 26 |
| 11 | 12.11 | 26 |
Table 7: Anti Bacterial activity of ligands and Metal Complexes.
The authors are grateful to Chairman, Department of Chemistry, Quaid-e- Azam University, Islamabad, Pakistan for synthesis of compounds and spectral studies.