Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2013) Volume 2, Issue 3
A series of Schiff bases and their derivative (oxazepine) have been synthesized. 1, 4-Bis (3-aminopropyl)- piperazine was condensed with various aromatic aldehyde in ethanol in the presence of acetic acid as catalyst to yield the Schiff bases. These Schiff’s bases on treatment with phthalic anhydride gave substituted oxazepine. The structure of synthesized Schiff bases has been established on the basis of their spectral (FT-IR, Mass, 1H, 13C-NMR, elemental analysis) data. The purity of the compounds was confirmed by TLC. The biological activities of some prepared compounds were also studied against four different kinds of bacteria. These compounds were tested to determine their ability to inhibit corrosion of mild steel in 1 mol.l-1 H2SO4.
Keywords: Schiff bases; 1, 4-Bis (3-aminopropyl)-piperazine; Oxazepine; Antibacterial activity; Anticorrosion; Mild steel
The development of simple synthesis route to widely used organic compounds ring, using readily available reagents is one of the main objectives of organic synthesis. Nitrogen heterocyclic are of a special interest because they constitute an important class of natural and non natural products, many of which exhibit useful biological activities, one–pot efficient synthesis of heterocyclic derivatives, may permit the development of novel therapies for the treatment of epilepsy, pain and other neurodegenerative disorder [1]. Some Schiff bases bearing aryl groups [2] or heterocyclic residues possess excellent biological activities [3], which has attracted many researchers’ attention in recent year. They have been reported to be used as analgesic, anthelmintic, antituberculer, plant growth regulator, antiviral, antifungal and anticancer [4]. Oxazepine derivative was introduced in 1965 for use in relief of the psychoneuroses characterized by anxiety and tension; oxazepam is non-homologous seven member ring contain two hetero atoms (oxygen and nitrogen) [5]. Oxazepine compounds have medical and biological important and they have medicinal and pharmaceutical application. Among the wide chemical derivatives are a heteropolymer which have activity and effectiveness against cancer [6], they also are effective against fungi and bacteria [7]. It was found that some Oxazepine derivatives are considered a medical drug against the disease [8]. Oxazepine derivatives are found to be effective against anxiety and associated with schizophrenia [9] also found that (7-hydroxyamoxapine) is a pharmaceutical composite affecting the nervous center (CNS) [10]. A send (as example) is an antidepressant with a mild sedative component to its action in animals (omoxazepine) reduced the uptake of noradrenalin and serotonin and block ed.
The response of dopamine receptors to dopamine [11]. These interesting biological activities attracted our attention to the chemistry of nitrogen heterocycles. Some oxazepine derivatives act as inhibitors of some enzymes action [12].
Several Schiff bases have recently been investigated as corrosion inhibitors for various metals and alloys in acid media. These substances generally become effective by adsorption on the metal surface. The adsorbed species protect the metal from the aggressive medium, which causes decomposition of the metal, but also on the chemical structure of the inhibitor [13-16].
In this work, the inhibiting action of Schiff bases and their derivative on the corrosion steel in 1M H2SO4 solution has been investigated. The electro chemical techniques such as polarization measurements were used in this study. Differences in behavior of inhibitors were explained based on structural properties of investigated inhibitors.
General procedures
Melting points were determined in open glass capillaries on agallenkamp apparatus and are uncorrected. TLC was performed to assess the reactions and the purity of the products using glass plates coated with silica gel of 0.25 mm thickness using mixture of petroleum ether/ethyl acetate (7:3; 6:4; 5:5 by V/V) as mobile phase Spots were visualized using iodine chamber and UV light chamber.
IR spectra were recorded in KBr (pellet forms) on aNicolet- Avatar-330 FT-IR spectrophotometer and note worthy absorption values (cm-1) alone are listed.1H and 13C NMR Spectra were recorded at 400 MHz Bruker AMX using CDCl3 as solvent. The ESI+ve MS spectra were recorded on a Bruker Daltonics LC-MS Spectrometer. Satisfactory microanalysis was obtained on Carlo Erba 1106 CHN analyzer. Potantiostat – galvanostat from Amel instruments where used for corrosion activity.
Chemical and starting materials
5-bromosalicylaldehyde, anthracene-9-carbaldehyde, 3-hydroxybenzaldhyde, 4-hydroxybenzaldhyde, O-vanilin, 4-chlorobenzaldhyde, 4-methylbenzaldhyde, 4-(dimethylamino) benzaldehyde and N,N’-bis (3-aminopropyl) piperazine (all from Aldrich) were used as supplied, without further purification.
General procedure for synthesis of schiff base and its derivatives
Preparation of Schiff bases (1-8): A series of Schiff bases were prepared from the reaction of 1,4-Bis (3-aminopropyl)-piperazine (1 mole), with different aldehydes (2 moles), in 20 ml ethanol absolute and few drops of glacial acetic acid. This mixture was refluxed for 2 hours. The mixture was cooled the precipitate was filtered and recrystallized from 1,4-dioxane [17,18].
(3, 3’-(piperazine-1, 4-diyl) bis (propane-3, 1-diyl)) bis (azan-1- yl-1-ylidene) bis (methan-1-yl-1-ylidene) bis (4-bromophenol) (1)
M.P (°C): 107-108, yield: 70%, M.F: C24H30Br2N4O2, M.Wt: 566, Elemental Analysis: Calculated: N%: 9.89, H: 5.34, C: 50.90, Found: N%: 9.87, H: 5.32, C: 50.89
IR (Nujol, cm-1): 1635 [υ(C=N)], 1163(s) [υ(C-O)]
MS (EI): m/z =566
1H NMR (400 MHz, CDCl3, ppm) δH: 1.81 (m, 4H, 9-H), 2.33- 2.40 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 6.79-7.31 (m, 6H, aromatic ring), 8.20 (s, 2H, 7-H, -C=N), 13.49 (b s, 2H, -OH)
13C NMR (400 MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.2 (C-11), 55.8 (C-10), 57.4 (C-8), 109.8 (C-6), 119.1 (C-5), 120.1 (C-2), 153.2, 154.8 (C-3 or C-4),160.5 (C-5) (aromatic ring), 163.8 (C-7, -C=N)
(anthracen-9-ylmethylene)-3-(4-(3-((E)-anthracen-9- ylmethyleneamino) propyl) pip erazin-1-yl) propan-1-amine (2)
M.P (°C): 168-170, yield: 79%, M.F: C40H40N4, M.Wt: 576.77, Elemental Analysis: Calculated N%: 9.71, H: 6.99, C: 83.30, Found: N%: 9.76, H: 6.95, C: 83.29
IR (Nujol, cm-1): 1635 [υ(C=N)], 1163(s) [υ(C-O)]
LC-MS: 576
1H NMR (400 MHz, CDCl3, ppm) δH: 1.81 (m, 4H, 9-H), 2.33- 2.40 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 7.39, 7.91, 8.55 (m, 18H, aromatic ring), 8.12 (s, 2H, 7-H, -CH=N)
13C-NMR (400 MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.2 (C-11), 55.8 (C-10), 57.4 (C-8), 135 (C-6), 125.6, 128.1, 128.9, 128.7, 131.8, 135 (26C, aromatic ring), 160.8 (C-7, -C=N)
(Chlorobenzylidene)-3-(4-(3-((E)-4-chlorobenzylideneamino) propyl) piperazin-1-yl) propan-1-amine (3)
M.P (°C): 105-106, yield: 52%, M.F: C24H30Cl2N4, M.Wt: 445, Elemental Analysis: Calculated: N%: 12.56, H: 6.79, C: 64.71, Found: N%: 12.46, H: 6.76, C: 64.69
IR (Nujol, cm-1): 1635 [υ(C=N)], 1163(s) [υ(C-O)]
LC-MS: 445
1H NMR (400 MHz, CDCl3, ppm) δH: 1.80 (m, 4H, 9-H), 2.46 (m, 12H, 10-H and 11-H), 3.55 (t(3J=8.0 Hz), 4H, 8-H), 7.07-7.31 (m, 8H, aromatic ring), 8.30 (s, 2H, 7-H)
13C-NMR (400 MHz, CDCl3, ppm): δC: 29.8(C-9), 52.9(C-11), 51.7(C-10), 58.1(C-8), 118.9(C-6), 116.9, 118.3, 128.9, 130.6(C-1, C-2, C-4, C-5), 136.6(C-3), 164.9(C-7, C=N)
4.3.5 3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (azan- 1-yl-1-ylidene) bis (methan-1-yl-1-lidene) bis (N, N-dimethylaniline) (4)
M.P (°C): 154, M.F: C28H42N6, M.Wt: 462.67, Elemental Analysis: Calculated: N%: 18.16, H: 9.15, C: 72.69, Found: N%: 18.17, H: 9.13, C: 72.70
IR (Nujol, cm-1): 1636.3 [υ(C=N)], 2930.31(s) [υ(C-H aliphatic)]
LC-MS: 462.67
1H NMR (400 MHz, CDCl3, ppm) δH: 1.80 (m, 4H, 9-H), 2.46 (m, 12H, 10-H and 11-H), 3.02 (s, 6H, 2(CH3)2N), 3.55 (t(3J=8.0 Hz), 4H, 8-H), 7.07-7.31 (m, 8H, aromatic ring), 8.30 (s, 2H, 7-H)
13C-NMR (400 MHz, CDCl3, ppm) δC: 29.9 (C-9), 41.2 (2CH3), 53.2 (C-11), 52.7 (C-10), 59.1 (C-8), 119.5 (C-6) 116.5, 118.2, 128.9, 131.3 (C-1, C-2, C-4, C-5), 151.9 (C-3), 164.9 (C-7, C=N)
(4-methylbenzylidene)-3-(4-(3-((E)-4-methylbenzylideneamino) propyl) piperazin-1-yl) propan-1-amine (5)
M.P (°C): 102-100, yield: 80%, M.F: C26H36N4, M.Wt: 404, Elemental Analysis: Calculated: N%: 13.85, H: 8.97, C: 77.18, Found: N%: 13.80, H: 8.96, C: 77.24
IR (Nujol, cm-1): 1635 [υ(C=N)], 1163(s) [υ(C-O)]
LC-MS: 404.5
1H NMR (400MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.41 (s, 6H, 2CH3), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 6.79-7.31 (m, 8H, aromatic ring), 8.33 (s, 2H, 7-H)
13C NMR (400 MHz, CDCl3, ppm) δC: 28.8 (C-9), 56.1 (C-11), 56.7 (C-10), 58.3 (C-8), 118.9 (C-6), 116.9, 118.3, 131.0, 132.0 (C-1, C-2, C-4, C-5), 140.7 (C-3) (aromatic ring), 164.5 (C-7, -C=N)
3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (azan-1-yl- 1-ylidene) bis (methan-1-yl-1-ylidene) diphenol (6)
M.P (°C): 110-111, yield: 80%, M.F: C24H32N4O2, M.Wt: 408, Elemental Analysis: Calculated: N%: 13.71, H: 7.90, C: 70.56, Found: N%: 13.69, H: 7.69, C: 70.53
IR (Nujol, cm-1): 1634 [υ(C=N)], 1163(s) [υ(C-O)]
MS (EI): m/z =408
LC-MS: 408.5
1H NMR (400 MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 6.79-7.31 (m, 8H, aromatic ring), 8.33 (s, 2H, 7-H), 13.51 (s, 2H, -OH)
13C NMR (400 MHz, CDCl3, ppm) δH: 27.8 (C-9), 53.1 (C-11), 55.7 (C-10), 57.3 (C-8), 118.8 (C-6), 116.9, 118.3, 131.0, 132.0 (C-1, C-2, C-3, C-5), 161.3 (C-2) (aromatic ring), 164.9 (C-7, -C=N)
(3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (azan-1- yl-1-ylidene) bis (methan-1-yl-1-ylidene) bis (2-methoxyphenol) (7)
M.P (°C): 102, yield: 60%, M.F C26H36N4O4, M.Wt: 468.59, Elemental Analysis: Calculated: N%: 11.96, H: 7.74, C: 66.64, Found: N%: 11.95, H: 7.72, C: 66.60
IR (Nujol, cm-1): 3300.65 [O-H], 1632.45 [υ(C=N)], 1156.12 [υ(C-O)]
LC-MS: m/z =468.59
1H NMR (400 MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 3.83 (s, 6H, 2OCH3), 6.79-7.31 (m, 6H, aromatic ring), 8.13 (s, 2H, 7-H), 9.83 (s, 2H, -2OH)
13C NMR (400MHz, CDCl3, ppm): 27.8(C-9), 53.1(C-11), 55.7(C- 10), 56.1(-2OCH3) 57.3(C-8), 118.8 (C-6), 116.9, 118.3, 131.0, 132.0 (C-3, C-5, C-4), 150.1(C-1), 151.2(C-2), 151.5(C-2) (aromatic ring), 165.9(C-7, -C=N)
(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (azan-1-yl-1- ylidene) bis (methan-1-yl-1-ylidene) diphenol (8)
M.P (°C): 93-94, yield: 79%, M.F: C24H32N4O2, M.Wt: 408, Elemental Analysis: Calculated: N%: 13.71, H: 7.90, C: 70.56, Found: N%: 13.69, H: 7.89, C: 70.55
IR (Nujol, cm-1): 3056.62 (υ(OH)), 1644.02 [υ(C=N)], 1163(s) [υ(C-O)]
LC-MS: m/z =408
1H NMR (400MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 6.79-7.31 (m, 8H, aromatic ring), 8.33 (s, 2H, 7-H), 13.51 (b s, 2H, -OH)
13C NMR (400 MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.1 (C-11), 55.7 (C-10), 57.3(C-8), 118.8 (C-6), 116.9, 118.3, 131.0, 132.0 (C-1, C-2, C-3, C-5), 161.3(C-3) (aromatic ring), 164.9 (C-7, -C=N)
Preparation of oxazepine (9-16)
A mixture of Schiff base (0.012 mole) and phthalic anhydride (0.025 mole) was dissolved in (20 mL) dry benzene. The mixture was heated for 5 hours in water bath at (70°C), excess solvent was distilled, the precipitate was filtered and recrystallized from ethanol [12,18].
(3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-(5-bromo-2-hydroxyphenyl)-3,4-dihydrobenzo[e] oxazepine- 1,5-dione:(9)) [1,3]
M.P (°C): 164, yield: 70%, M.F: C40H38Br2N4O8, M.Wt: 862, Elemental Analysis: Calculated: N%: 6.50, H: 4.44, C: 55.70, Found: N%: 6.51, H: 4.40, C: 55.69
IR (cm-1): 3284.18 [υ (OH)], 1709.59 [υ (C=O lactones)], 1634.38 [υ (C=O) lactic], 1381.75 (O-C-O)
MS (EI): m/z=862.56
1H NMR (400 MHz, CDCl3, ppm) δH: 1.81 (m, 4H, 9-H), 2.33- 2.40 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 6.79-7.31 (m, 6H, aromatic ring), 7.35 (s, 2H, N-CH-O), 13.49 (s, 2H, -OH), 7.62- 8.15 (m, 8H, aromatic (oxazepine)
13C NMR (400 MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.2 (C-11), 55.8 (C-10), 57.4 (C-8), 84.2 (N-CH) 109.8 (C-6), 119.1 (C-5), 120.1 (C-2), 132.9, 132.1, 129.7, 127.1 (12C, aromatic of pthalic anhydride), 153.2, 154.8 (C-3 or C-4)160.5 (C-5) (aromatic ring), 162, 169.9 (2C=O of lactone and lactam respectively).
(3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-(anthracen-9-yl)-3,4-dihydrobenzo[e] oxazepine-1,5-dione (10) [1,3]
M.P (°C): 148-152, yield: 69, M.F C56H50N4O5, M.Wt: 85, Elemental Analysis: Calculated: N%: 6.52, H: 5.87, C: 78.3 Found: N%: 6.50, H: 5.85, C: 78.29
IR: 3120(C-H aromatic), 2929.15 (C-H aliphatic), 1707.60 [υ (C=O) lactone], 1637.45 [υ (C=O) lactam], 1375.55 (O-C-O)
LC-MS: 873
1H NMR (400 MHz, CDCl3, ppm) δH: 1.81 (m, 4H, 9-H), 2.33- 2.40 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 7.35 (s, 2H, N-CH-O), 7.91, 8.55 (m, 18H, aromatic ring), 7.35 (s, 2H, -CH=N), 7.62, 7.78, 8.15 (m, 8H, aromatic carbons )
13C-NMR (400 MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.2 (C-11), 55.8 (C-10), 57.4 (C-8), 82.5 (N-CH), 135 (C-6), 125.6, 128.1, 128.9, 128.7, 131.8, 135 (26C, aromatic ring), 161.2, 168.5 (2C=O, of lactone and lactam respectively), 127.1, 129.7, 132.1, 132.9 (12C, aromatic ring of phthalic anhydride)
(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-(4-chlorophenyl)-3,4-dihyd robenzo[e] oxazepine-1,5-dione) (11) [1,3]
M.P (°C): 182, yield: 80, M.F: C40H40Cl2N4O5, M.Wt: 727, Elemental Analysis: Calculated: N%: 7.70, H: 5.54, C: 66.02, Found: N%: 7.69, H: 5.53, C: 65.95
IR: 3011.52 (C-H aromatic), 2937.35 (C-H aliphatic), 1709.60 [υ(C=O) lactone), 1647.45 [υ(C=O) lactam], 1166.98 (Ar-Cl)
LC-MS: 741.66
1H NMR (400 MHz, CDCl3, ppm) δH: 1.80 (m, 4H, 9-H), 2.46 (m, 12H, 10-H and 11-H), 3.55 (t, (3J=8.0 Hz), 4H, 8-H), 7.35 (s, 2H, N-CH-O), 7.07-7.31 (m, 8H, aromatic ring), 7.62, 7.78, 7.81, 8.15 )m, 4H, aromatic ring of phthalic anhydride)
13C-NMR (400 MHz, CDCl3, ppm) δC: 29.8 (C-9), 52.9 (C-11), 51.7 (C-10), 58.1 (C-8), 84.2 (C-7, C=N), 118.9 (C-6), 116.9, 118.3, 128.9, 130.6 (C-1, C-2, C-4, C-5), 136.6 (C-3), 127.1, 129.7, 132.1, 132.9 (12C, aromatic of phthalic anhydride), 161.2, 167 (2C=O, of lactone and lactam respectively)
(3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-(4-(dimethylamino)phenyl)-3,4-dihydrobenzo[e]oxazepine-1,5- dione (12) [1,3]
M.P (°C): 186, yield: 79, M.F: C44H52N6O5, M.Wt: 744, Elemental Analysis: Calculated: N%: 11.28, H: 7.04, C: 70.94, Found: N%: 11.27, H: 7.03, C: 70.93
IR: 3015.74 (C-H aromatic), 2985.75 (C-H aliphatic), 1710.61 [υ(C=O) Lactone], 1696.49 [υ(C=O) lactam], 1581 (C-N)
LC-MS: 759
1H NMR (400 MHz, CDCl3, ppm) δH: 1.80 (m, 4H, 9-H), 2.46 (m, 12H, 10-H and 11-H), 3.02 (s, 6H, 2(CH3)2N), 3.55 (t(3J=8.0 Hz), 4H, 8-H), 7.07-7.31 (m, 8H, aromatic ring), 7.35 (s, 2H, N-CH), 7.62, 7.78, 7.81, 8.16 (m, 4H, aromatic of phthalic anhydride)
13C-NMR (400 MHz, CDCl3, ppm) δC: 29.9(C-9), 41.2 (2CH3), 53.2 (C-11), 52.7 (C-10), 59.1 (C-8), 84.2 (N-CH) 119.5 (C-6) 116.5, 118.2, 128.9, 131.3 (C-1, C-2, C-4, C-5), 151.9 (C-3), 127.1, 129.7, 132.9, 132.1 (12C, aromatic carbons of phthalic anhydride), 161.2, 167 (2C, 2C=O of lactone and lactam respectively)
3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-p-tolyl- 3,4-dihydrobenzo[e]oxazepine-1,5-dione (13) [1,3]
M.P (°C): 154, yield: 74, M.F C42H46N4O5, M.Wt: 686.84, Elemental Analysis: Calculated: N%: 8.16, H: 6.75, C: 73.45, Found: N%: 8.15, H: 6.74, C: 73.40
IR: 3035.74 (C-H aromatic), 2996.75 (C-H aliphatic), 1718.69 [υ (C=O) lactone], 1698.78 [υ (C=O) lactam]
LC-MS: 700
1H NMR (400MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.41(s, 6H, 2CH3), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 6.79-7.31 (m, 8H, aromatic ring), 7.35 (s, 2H, N-CH), 7.62, 7.78, 7.81, 8.15 (m, 8H, aromatic ring of phthalic anhydride)
13C NMR (400 MHz, CDCl3, ppm) δC: 28.8 (C-9), 56.1 (C-11), 56.7 (C-10), 58.3 (C-8), 118.9 (C-6), 116.9, 118.3, 131.0, 132.0 (C-1, C-2, C-4, C-5), 140.7 (C-3) (aromatic ring), 84.2 (N-CH), 127.1, 129.7, 132.9 (12C, aromatic ring of phthalic anhydride), 161.2, 167 (2C, 2C=O of lactone and lactam respectively).
3,3’-piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-(3-hydroxyphenyl)-3,4-dihydrobenzo[e]oxazepine-1,5-dione (14) [1,3]
M.P (°C): 147, yield: 86, M.F: C40H40N4O8, M.Wt: 704, Elemental Analysis: Calculated: N%: 7.95, H: 5.72, C: 68.17, Found: N%: 7.94, H: 5.69, C: 68.16
IR: 3360 (OH), 3028 (C-H aromatic), 2872 (C-H aliphatic), 1715.61 [υ (C=O) lactone], 1696.49 [υ (C=O) lactam],
LC-MS: 704.77
1H NMR (400 MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 7.35 (s, 2H, N-CH), 6.79-7.31 (m, 8H, aromatic ring), 7.62, 7.78, 7.81, 8.45 (m, 4H, aromatic phthalic anhydride), 13.51 (s, 2H, -OH).
13C NMR (400MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.1 (C-11), 55.7 (C-10), 57.3 (C-8), 84.5 (N-CH), 118.8 (C-6), 116.9, 118.3, 131.0, 132.0 (C-1, C-2, C-3, C-5), 161.3 (C-2) (aromatic ring), 127.1, 129.7, 132.9 (aromatic carbons of phthalic anhydride), 161.2, 167.2 (2C, 2C=O of lactone and lactam respectively)
(3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-(2-hydroxy-3-methoxyphenyl)-3,4-dihydrobenzo[e] oxazepine- 1,5-dione) (15) [1,3]
M.P (°C): 168, yield: 72, M.F: C42H44N4O10, M.Wt: 764.82, Elemental Analysis: Calculated: N%: 7.33, H: 5.80, C: 65.96, Found: N%: 7.32, H: 5.79, C: 65.95
IR: 3371(OH), 3056 (C-H aromatic), 2950 (C-H aliphatic), 1748.93 [υ (C=O) lactone], 1698.49 [υ (C=O) lactam],
LC-MS: 764.82
1H NMR (400 MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 3.83 (s, 6H, 2OCH3), , 6.79-7.31 (m, 6H, aromatic ring), 7.35 (s, 2H, CH-N), 9.83 (s, 2H, -2OH), 7.62, 7.78, 7.81, 8.15(m, 4H, aromatic rig of phthalic anhydride)
13C NMR (400MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.1 (C-11), 55.7 (C-10), 56.1 (-2OCH3) 57.3 (C-8), 78.3 (N-CH), 118.8 (C-6), 116.9, 118.3, 131.0, 132.0 (C-3, C-5, C-4), 150.1 (C-1), 151.2 (C-2), 151.5 (C-2) (aromatic ring), 127.1, 129.7, 132.1, 132.9 (aromatic carbons of phthalic anhydride), 161.2, 167 (2C, 2C=O)
3,3’-(piperazine-1,4-diyl) bis (propane-3,1-diyl)) bis (3-(4-hydroxyphenyl)-3,4-dihydrobenzo[e] oxazepine-1,5-dione) (16) [1,3]
M.P (°C): 161, yield: 73, M.F: C40H40N4O8, M.Wt: 704, Elemental Analysis: Calculated: N%: 7.95, H: 5.72, C: 68.17, Found: N%: 7.93, H: 5.71, C: 68.13
IR: 3360(OH), 3072 (C-H aromatic), 2956 (C-H aliphatic), 1749.03 [υ (C=O) Lactone], 1699.05 [υ (C=O) lactam]
LC-MS: 704.77
1H NMR (400MHz, CDCl3, ppm) δH: 1.87 (m, 4H, 9-H), 2.47 (m, 12H, 10-H and 11-H), 3.58 (t(3J=8.0 Hz), 4H, 8-H), 6.79-7.31 (m, 8H, aromatic ring), 7.35 (s, 2H, CH-N), 7.62, 7.78, 7.81, 8.15 (m, 4H, aromatic ring of phthalic anhydride), 13.51 (b s, 2H, -OH)
13C NMR (400 MHz, CDCl3, ppm) δC: 27.8 (C-9), 53.1 (C-11), 55.7 (C-10), 57.3 (C-8), 84.2 (CH-N), 118.8 (C-6), 116.9, 118.3, 131.0, 132.0 (C-1, C-2, C-3, C-5), 161.3 (C-3) (aromatic ring), 127.1, 129.7, 132.1, 132.9 (aromatic carbons of phthalic anhydride), 161.2, 167(2C, 2C=O)
All newly synthesized compounds were tested for their in vitro growth inhibitory activity against a standard strain of pathogenic microorganism including Gram–positive bacteria (Staphylococcus aureus), Gram - negative bacteria (Escherichia coli, Bacillus). Antibacterial activity was done by the disk diffusion method S.aureus and E.coli were sub cultured in BHI medium and incubated for 18 hours at 37°C, and then the bacterial cells were suspended, according to the McFarland protocol in saline solution to produce a suspended of about 10-5 CFU ml-1. 10 μl of this suspension was mixed with 10 ml of sterile antibiotic agar at 40°C and poured onto an agar plate in laminar flow cabinet. Five paper disks (6.0 mm diameter) were fixed onto nutrient agar plate 0.1 mg of each test compound was dissolved in 100 DMSO to prepare stock solution from stock solution different concentration 100, 250, 500, 1000 ppm of each test compound were prepared. These compounds of different concentration were poured over disk plate on to it. Streptomycin was used as standard drug (positive control) DMSO poured disk was determined by the formation of an inhibitory zone after 24 hours of incubation at 36°C. Table 1 reports the inhibitory zones (mm) of each compound and the controls [19].
| Comp. No. | S. aureus | E. coli | S. typhi | K. pneumonia |
|---|---|---|---|---|
| 9 | ± | ± | ++ | ++ |
| 10 | ± | ± | ++ | ++ |
| 11 | ± | ± | ++ | ++ |
| 12 | ± | ++ | ± | ++ |
| 13 | ± | ++ | ± | ++ |
| 14 | ++ | ++ | ++ | ± |
| 15 | ++ | ++ | ++ | ± |
| 16 | ++ | ++ | ++ | ± |
Key the symbols :(-) = No inhibition, (±) = 6-9 mm, (++) = 15-22 mm
Table 1: Antimicrobial activity for prepared compounds.
Electrochemical measurements were carried out in conventional three –electrode system in CHI 604 instrument (USA) at 303 K. The working electrode (mild steel) has a geometric area of 1 cm2.The saturated calomel and platinum electrodes were used as reference and auxiliary electrodes. Equation (1) shows the calculation of IE from corrosion current [20-24]:
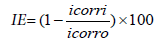 (1)
(1)
Chemistry and characterization
The present work involved two steps:
First step: It includes preparation of new eight Schiff bases. (1-8) were prepared by reaction of 1,4 bis (3-aminopropyl)-piperazine with different aldehydes. The synthesis of these compounds was carried out lined in Scheme (1) and the physical properties for Schiff bases (1-6) including melting point range of (100-170) and % yield were range of (70-80) and these compounds were identified by FT-IR Spectroscopy, Lc-MS, 1H-, 13C-NMR. FT-IR spectrum of compounds (1-6) showed characteristic absorption bands (1635) cm-1, (3140) cm-1, (2885-2997) cm-1 due to Ê?(C=N), Ê?(C-H) aromatic, Ê?(C-H) aliphatic respectively. 1H-NMR spectrum of compound (7) showed multiple signals at (6.79- 7.31) ppm due to aromatic protons and singlet signal at (8.20 ppm) due to (C-H) group and singlet signal at 13.49 ppm due to (OH) group in addition to multiple signals at (1.81) due to (C-H) (9) and multiple signals at (2.33-2.40) ppm due to (C-H)(11), (C-H)(10) and triplet signal at (3.58 ppm) due to (C-H) (8). 1H-NMR of compound (8) show multiple signals at (7.39, 7.91, 8.55 ppm) due to aromatic protons of anthracene.
Second step: The second step included preparation of new eight Oxazepine. (9-16) were prepared by reaction of Schiff bases (1-8) in (first step) with phthalic anhydride in dry benzene The synthesis of these compounds was carried out lined in scheme (1) and the physical properties for oxazepine (9-16) including melting point range of (150- 186) °C and %Yield were range (70-80) and these compounds were identified by FT-IR, LC-MS and 1H, 13C-NMR and FT-IR spectrum of compounds (9-16) showed clear absorption bands at (1634.38) cm-1 and (1709.59) cm-1 attributed to the Ê?(C=O) of lactone and lactam inside oxazepine ring. The 1H-NMR spectrum of compound (9-16), showed multiple signals at (7.62-8.15) ppm due to aromatic protons of oxazepine ring and a singlet signal at (7.35) ppm due to N- CH-O group of oxazepine ring, 13C-NMR spectrum of compounds (9-16) showed signals at (127.1, 129.7, 132.1, 132.9) ppm due to aromatic carbons and signals at (162-167) ppm due to (C=O) of lactone and lactam respectively, signals at (84.2) ppm due to (N-CH-O) carbon [23,24].
Antimicrobial activity
The newly synthesized compounds were screened for their antibacterial activity against Escherichia coli, Staphylococcus aureus, Salmonella typhii, Klebsiella pneumonia. The results of such studies are given in Table 1. The above data showed that compounds [14-16] exhibited very good activity against E.coli, S.aureus, Salmonella typhii, and moderate activity against Klebsiella pneumonia. The compounds [9-13] show good activity agaist Klebsiella pneumonia and moderate activity against S.aureus and E.coli.
Polarisation measurements
Table 2 shows the corrosion Potential (Ecorr), corrosion current (icorr) and Tafel slopes (ba and bc) values of mild steel in 1 mol.L-1 H2SO4. Solution in the absence and presence of inhibitor of all the eight compounds at 303K calculated from Scheme [2-9]. From Table 2 it is clear that compound (10,14,16) offers maximum inhibition efficiency among the eight compounds Schiff base derivatives and the studied compounds suppress the anodic reaction to greater extent than the cathodic one. This behavior is typical of mixed type inhibitors with anodic predominance.
| IE(Using icorr) % | Rp | bc /(mv.dec -1) |
ba /(mv.dec -1) | icorr/µA cm1- | -Ecorr/mv | COMPOUND Name |
|---|---|---|---|---|---|---|
| BLank | 12.43 | 6.38 | 480 | 480 | blank | |
| 62.70 | 102.95 | 27.49 | 5.019 | 179 | 400 | 9 |
| 89.58 | 496.27 | 14.55 | 10.09 | 50 | 520 | 10 |
| 63.95 | 95.614 | 25.78 | 4.47 | 173 | 420 | 11 |
| 63.54 | 95.95 | 25.78 | 4.55 | 175 | 418 | 12 |
| 58.54 | 71.13 | 19.52 | 6.28 | 199 | 450 | 13 |
| 85.16 | 330.54 | 11.17 | 10.19 | 70 | 500 | 14 |
| 79.16 | 221.58 | 14.55 | 7.86 | 100 | 510 | 15 |
| 87.5 | 349.83 | 11.82 | 8.18 | 60 | 520 | 16 |
Table 2: Corrosion kinetic parameters of mild steel exposed to 1 mol.L-1H2SO4 solution in absence and presence of inhibitors.
The difference in the efficiency is referred to the molecular structure effect, which have π-delocalized system of Schiff bases derivatives (C=O) and unshared pairs of electrons of N and O atoms that may cause the increasing or decreasing of the electron density on center of adsorption and leading to an easier electron transfer from the functional group (C=O group) to the metal, producing grater coordinate bonding and hence different adsorption and inhibition efficiency.
Mechanism of corrosion inhibition by Schiff base derivatives: The transition of metal/solution interface from a state of active dissolution to the passive state is attributed to the adsorption of the inhibitor molecules and the metal surface, forming a protective film. The rate of adsorption is usually rapid and hence, the reactive metal surface is shielded from the aggressive environment, Adsorption process can occur by electrostatic forces between ionic charges or dipoles of the adsorbed species (electrostatic attraction between the positively charged protonated nitrogen atom and negatively charged mild steel surface, cathodic sites) and the electric charge on the metal surface can be expressed by its potential with respect to the zero charge potential, Also the inhibitor molecules can be adsorbed species to the vacant electron orbital of low energy in the metal to form a coordinate type of link. Adsorption of inhibitor molecules is often a displacement reaction involving removal of adsorbed water molecules is often adisplacement reaction involving removal of adsorbed water molecules from the metal surface.
The main aim of the present study is to synthesize and investigate the antimicrobial and anti corrosion activity of new heterocyclic derivatives containing, oxazepine ring with the hope of discovering new structures serving as potential broad spectrum antimicrobial agents and anti corrosion agents, the antibacterial revealed that nature of substituents on the phenyl ring viz., methyl, methoxy, hydroxyl, dimethylamin, chloro, bromo and hydroxyl group at the para positions of the aryl moieties are determinant for the nature and extent of the anti-bacterial activity of the synthesized compounds, which might have influences on their inhibiting mechanism of actions. Compound (13- 16) which contain electron donating functional moiety is most potent against bacterial it has showed good antimicrobial activity. From the results, it is obvious that all eight studied compounds function as effective corrosion inhibitors in 1 mol.L-1 H2SO4 medium with compounds 10, 14 and 16 being the best of eight compounds.