Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2014) Volume 6, Issue 1
Citral (3, 7-dimethyl-2, 6-octadienal)was hydro distilled from lemon grass and 3, 7-dimethyl-2, 6-octadienal acetals (Citral acetals) were synthesized from it. The latter was redistilled under vacuum and collected fractions at constant temperature. Para toluene sulphonic acid catalyst was used for the preparation of citral ethylene glycol acetal and citral propylene glycol acetal which are used in perfumery, flavor, for fortifying lemon oil. GC, IR of synthesized product is recorded and graphs were determined.
<Citral is present in several plant oils like lemon myrtle, Litsea citrata, lemon grass, lemon tea tree, Ocmum gratissimum and lemon balm etc., [1]. Citral, or 3, 7-dimethyl-2, 6-octadienal or lemonal, is either of, or a mixture of, a pair of mono terpenoidswith the molecular formula C10H16O [2] the two compounds are diastereo-isomers. The E-isomer is known as geranial or citral A. The Z-isomeris known as neral or citral B [3] (Figure 1). Commercial Citral, invariably, is a mixture of two isomers due to cis–trans isomerism at the C=C bond nearly the aldehyde group obtained from essentials oils of plant sources. It has lemon odour. The isomer geranial has strong lemon odour. The isomer neral has lemon odour is less strong and sweeter. Citral is, therefore, an aromatic compound used in perfumery for its citrus effect. Citral is also used as a flavour and for fortifying lemon oil. It also has strong anti-microbial qualities [4] and pheromonal effects in insects [5]. Citrals are basic intermediate for synthesis of flavouring and fragrance components such as Ionones, methyl ionones and vitamins A and E [6,7].
Citral is a perfume having a strong lemon-like aroma contained in natural lemon and lemon grass essential oils, but it is highly volatile and unstable to air, sunrays and alkalis, thus hardly sustaining its aroma. To solve this problem, citral dimethyl, acetal and citral diethyl acetal have been used, but these compounds possess not lemon aromas but nerolilike citrus green aromas, and do thus not possess the lemon-like aroma of citral. Most glycosidic bonds in carbohydrates and polysaccharides are acetal linkage [8] and acetaldehyde diethyl acetal is an important flavouring compound in beverages [9]. Citral propylene glycol acetal using propylene glycol having a weak aroma, but it was recognized that this acetal itself is volatile and has a green aroma, thus inhibiting the lemon aroma unique to citral. Citral mono ether glyceryl acetals and citral propylene glycol acetals are also synthesized and used in cosmetics and toiletries to suppress body smell produce by bacteria instead antibacterial agents.Our aim is to make acetals of citral with ethylene glycol and propylene- glycol. The formation of Citral acetals from citral and glycols is acid catalysed reaction and water thus produce during the reaction is continuously removed by azeotropic distillation. Typical synthesis of citral acetals and important citral acetals used in cosmetics is shown in Figure 1.
Extraction of essential oils
Lemon grass leaves were obtained from PCSIRBotanical garden and dried under shade for two days and cut into small pieces for distillation. Lemon-grass oil was hydro-distilled in Dean Stark apparatus using 2 liter round bottomed flask. Three fourth of the flask was filled with 500 gm of crushed dried leaves of lemon grass along with water and hydro-distilled. The oil was separated and dried over anhydrous sodium sulfate. It yield gram of oil (0.38%). Hydro distillation was repeated to get required amount of citral which was redistilled under vacuum and collected the fractions between 110°C-117°C. Then GC, IR of this citral was recorded and graphs were appended in (Figures 2-8).
Preparation of citral propylene glycol acetal
To 16ml solution of citral was added 22 ml of propylene glycol, 60ml toluene and few crystals of para-toluene-sulfonic-acid (as catalyst) in 500 ml capacity round bottomed distillation flask fitted with dean stark apparatus and stirred under heating at 110°C. Water and toluene was azeotropically distilled off, the reaction residue is neutralized with sodium bicarbonate solution and extracted with benzene and dried over MgSO4. Benzene was distilled off and the residual product was distilled under vacuum and three fractions between 121-125°C citral propylene glycol. The fractions gave fruity odour. GC, IR of the fractions was recorded.
Citral ethylene glycol acetal
To 8ml solution of citral was added 11 ml of ethylene glycol, 10ml benzene along with few crystals of para toluene sulfonic acid in 250 ml capacity round bottomed distillation flask along with condenser and then stirred the mixture while heating. Water was formed during reaction which was removed as azeotrope mixture with benzene. The residual product was extracted with hexane and neutralize with sodium bicarbonate solution, washed with water and hexane layer was died over Magnesium carbonate. The hexane extract was distilled off. The left in the flask was distilled under vacuum, using still head and collected three fractions at different fractions. These fractions have thymol fruity odours. GC, IR is recorded and appended.
Interpretation of IR spectra of citral propylene glycol acetal (Table 1)
| Sr.# | Peaks | Intensity | Assignment | ||
|---|---|---|---|---|---|
| F1 | F2 | F3 | |||
| 1 | 3019 | 3019 | 3019 | Sharp&Strv.close | -CH3,CH2,C-H |
| 2 | 2925 | 2925 | 2925 | Sharp&Str | C=O |
| 3 | 2871 | 2871 | 2869 | Sharp | CH3,CH2,C-H |
| 4 | 1894 | Wk& sharp | isomers | ||
| 5 | 1744 | Wk& sharp | |||
| 6 | 1667 | Wk& sharp | -CH=CH- | ||
| 7 | 1608 | 1608 | Med | unsaturation | |
| 8 | 1514 | 1514 | 1514 | Sharp&med | C=C |
| 9 | 1460 | 1460 | 1460 | Sharp&Str | O-H |
| 10 | 1380 | 1380 | 13880 | Sharp&Str | 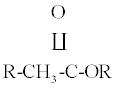 |
| 11 | 1108 | 1106 | 1106 | Str | |
| 12 | 1055 | 1055 | 1056 | 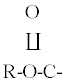 |
|
wk =weak, med=medium, stre=stretching
Table 1: IR data of citral propylene glycol acetal.
Fraction 1: An IR spectrum of fraction has strong characteristic peaks at 3019, 2925 and 2871 cm-1 which is specific for C-H stretching. The medium peaks at 1608, 1514 and 1460 cm-1 is characteristics for un-saturation C=C in the citral fragment of citral propylene glycol acetal.
Fraction 2: The IR Spectra of fraction 2 shows characteristic peaks at 3019, 2925, 2869, which is specific for C-H stretching. The medium peaks at 1667, 1514, 1460, 1380 are due to C=C stretching due to un-saturation in citral fragment in this compound. The stretching vibrations at 1056, 1106, are due to ether linkage in the given compound.
Fraction 3: Characteristic peaks at 3019, 2925, 2871 are specific for C-H stretching. The medium peaks at 1608, 1514, 1460, and 1308 are due to C=C stretching peaks at 1055, 814, 742 are stretching vibrations due to ether linkage in the compound.
Interpretation of GC data of citral propylene glycol acetal (Table 2)
| Sr.# | Compound name | % age Composition | |
|---|---|---|---|
| F1 | F2 | ||
| 1 | Citral propylene glycol Acetal | 55.4 | 31.2 |
| 2 | Citral(cis/trans) | 16.23 | 18.5 |
| 3 | UnIdentified | 6.5 | 10.3 |
| 4 | UnIdentified | 6.1 | 6.2 |
| 5 | Other isomers | 4.9 | 5.8 |
| 6 | UnIdentified | 4.5 | 4.2 |
| 7 | UnIdentified | 2.9 | 4.0 |
| 8 | UnIdentified | 1.2 | 3.5 |
| 9 | UnIdentified | 0.7 | 2.4 |
| 10 | UnIdentified | 0.4 | 2.1 |
| Total | 98.83 | 95.7 | |
Table 2: GC data of citral propylene glycol acetal.
The reaction mixture of citral propylene glycol acetal later extraction with benzene, redistilled under vacuum at 64-88°C Major components are shown in table 2 The order of redistilled compound is thymol rose. As the citral contained cis and trans geometrical isomers, it gave many product by reaction with propylene glycol, due to following reason.
The formation of citral propylene glycol acetal expected to give four more isomers due to formation of two asymmetric centres at C2 and C4 of 1,3-dioxo-4 methyl-citral acetal.
Interpretation of IR spectra of citral ethylene glycol acetal
The IR spectra of citral ethylene glycol acetal has characteristic peak at 2924 which shows C-H stretching. Peak at 2867 shows the presence of CH3, CH2, CH in the given compound. Peak at 1665 shows unsaturation in compound. Peak at 1055 shows the presence of C-O and at 814 shows C=C, other peak at 1513, 1460, 1379shows the presence of other isomers in the compound (Table 3).
| Sr.# | Peaks | Intensity | Assignment |
|---|---|---|---|
| 1 | 3747 | Wk | |
| 2 | 3649 | Med | C-H |
| 3 | 2924 | Sharp&Str | C-H |
| 4 | 2867 | Sharp&Str | CH3,CH2,CH |
| 5 | 1665 | Med | C=C |
| 6 | 1513 | Sharp&Str | C=O |
| 7 | 1460 | sharp | C-H |
| 8 | 1379 | Med | OH |
| 9 | 1055 | Med | 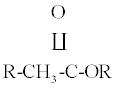 |
wk =weak, med=medium, stre=stretching
Table 3: IR Absorption spectra of Citral ethylene glycol acetal.
Interpretation of GC data of citral ethylene glycol acetal
Citral ethylene glycol acetal was subjected to GC analysis and percentage composition of major components was determined GC showed two peaks of citral ethylene glycol acetal which are two isomers, cis& Trans. This is justified as the starting citral has two geometrical isomers. Other isomers are also present in small amount (Table 4).
| Sr# | Compound name | %age composition |
|---|---|---|
| 1 | Citral ethylene glycol Acetal | 52.7 |
| 2 | (cis&trans) | 38.5 |
| 3 | Other isomers | 8.3 |
| 4 | 0.3 | |
| Total | 99.8 | |
Table 4: GC data for Citral ethylene glycol acetal.
Interpretation of IR spectra of citral propylene glycol acetal (Table 1)
| Sr.# | Peaks | Intensity | Assignment | ||
|---|---|---|---|---|---|
| F1 | F2 | F3 | |||
| 1 | 3019 | 3019 | 3019 | Sharp&Strv.close | -CH3,CH2,C-H |
| 2 | 2925 | 2925 | 2925 | Sharp&Str | C=O |
| 3 | 2871 | 2871 | 2869 | Sharp | CH3,CH2,C-H |
| 4 | 1894 | Wk& sharp | isomers | ||
| 5 | 1744 | Wk& sharp | |||
| 6 | 1667 | Wk& sharp | -CH=CH- | ||
| 7 | 1608 | 1608 | Med | unsaturation | |
| 8 | 1514 | 1514 | 1514 | Sharp&med | C=C |
| 9 | 1460 | 1460 | 1460 | Sharp&Str | O-H |
| 10 | 1380 | 1380 | 13880 | Sharp&Str | 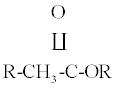 |
| 11 | 1108 | 1106 | 1106 | Str | |
| 12 | 1055 | 1055 | 1056 | 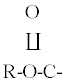 |
|
wk =weak, med=medium, stre=stretching
Table 1: IR data of citral propylene glycol acetal.
Fraction 1: An IR spectrum of fraction has strong characteristic peaks at 3019, 2925 and 2871 cm-1 which is specific for C-H stretching. The medium peaks at 1608, 1514 and 1460 cm-1 is characteristics for un-saturation C=C in the citral fragment of citral propylene glycol acetal.
Fraction 2: The IR Spectra of fraction 2 shows characteristic peaks at 3019, 2925, 2869, which is specific for C-H stretching. The medium peaks at 1667, 1514, 1460, 1380 are due to C=C stretching due to un-saturation in citral fragment in this compound. The stretching vibrations at 1056, 1106, are due to ether linkage in the given compound.
Fraction 3: Characteristic peaks at 3019, 2925, 2871 are specific for C-H stretching. The medium peaks at 1608, 1514, 1460, and 1308 are due to C=C stretching peaks at 1055, 814, 742 are stretching vibrations due to ether linkage in the compound.
Interpretation of GC data of citral propylene glycol acetal (Table 2)
| Sr.# | Compound name | % age Composition | |
|---|---|---|---|
| F1 | F2 | ||
| 1 | Citral propylene glycol Acetal | 55.4 | 31.2 |
| 2 | Citral(cis/trans) | 16.23 | 18.5 |
| 3 | UnIdentified | 6.5 | 10.3 |
| 4 | UnIdentified | 6.1 | 6.2 |
| 5 | Other isomers | 4.9 | 5.8 |
| 6 | UnIdentified | 4.5 | 4.2 |
| 7 | UnIdentified | 2.9 | 4.0 |
| 8 | UnIdentified | 1.2 | 3.5 |
| 9 | UnIdentified | 0.7 | 2.4 |
| 10 | UnIdentified | 0.4 | 2.1 |
| Total | 98.83 | 95.7 | |
Table 2: GC data of citral propylene glycol acetal.
The reaction mixture of citral propylene glycol acetal later extraction with benzene, redistilled under vacuum at 64-88°C Major components are shown in table 2 The order of redistilled compound is thymol rose. As the citral contained cis and trans geometrical isomers, it gave many product by reaction with propylene glycol, due to following reason.
The formation of citral propylene glycol acetal expected to give four more isomers due to formation of two asymmetric centres at C2 and C4 of 1,3-dioxo-4 methyl-citral acetal.
Interpretation of IR spectra of citral ethylene glycol acetal
The IR spectra of citral ethylene glycol acetal has characteristic peak at 2924 which shows C-H stretching. Peak at 2867 shows the presence of CH3, CH2, CH in the given compound. Peak at 1665 shows unsaturation in compound. Peak at 1055 shows the presence of C-O and at 814 shows C=C, other peak at 1513, 1460, 1379shows the presence of other isomers in the compound (Table 3).
| Sr.# | Peaks | Intensity | Assignment |
|---|---|---|---|
| 1 | 3747 | Wk | |
| 2 | 3649 | Med | C-H |
| 3 | 2924 | Sharp&Str | C-H |
| 4 | 2867 | Sharp&Str | CH3,CH2,CH |
| 5 | 1665 | Med | C=C |
| 6 | 1513 | Sharp&Str | C=O |
| 7 | 1460 | sharp | C-H |
| 8 | 1379 | Med | OH |
| 9 | 1055 | Med | 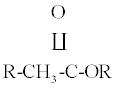 |
wk =weak, med=medium, stre=stretching
Table 3: IR Absorption spectra of Citral ethylene glycol acetal.
Interpretation of GC data of citral ethylene glycol acetal
Citral ethylene glycol acetal was subjected to GC analysis and percentage composition of major components was determined GC showed two peaks of citral ethylene glycol acetal which are two isomers, cis& Trans. This is justified as the starting citral has two geometrical isomers. Other isomers are also present in small amount (Table 4).
| Sr# | Compound name | %age composition |
|---|---|---|
| 1 | Citral ethylene glycol Acetal | 52.7 |
| 2 | (cis&trans) | 38.5 |
| 3 | Other isomers | 8.3 |
| 4 | 0.3 | |
| Total | 99.8 | |
Table 4: GC data for Citral ethylene glycol acetal.
Citral is an important intermediate chemical for the synthesis of flavours and vitamin A and vitamin E. Citral is obtained from fractional distillation of lemon-grass as well total synthesis from myrcene and 2-methy-4-hydroxy-but-1-ene on commercial scale. The citral acetals are also important intermediate for the synthesis of flavour as well as their use in perfume and cosmetics.it is also used as anti-bacterial agent because Citral acetals reduce the bacterial activity. Purification test and product like citral propylene glycol acetal and ethylene glycol acetal is synthesized and evaluated by GC and IR.