Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2012) Volume 1, Issue 1
A macrocyclic hydrazone Schiff bases were synthesized by reaction of pyridine-2,6-dicarbohydrazide and pyridine-2,6-thiodicarbohydrazide with dicarbonyls. Schiff bases have been characterized by melting point, elemental analyses, LC-MS, IR, 1 H and 13 C NMR spectral data. The Schiff base from pyridine-2,6-dicarbohydrazide and benzil has been studied by liquid–liquid extraction towards the d-metal ions (Cu(II) and Cr(III)) from aqueous phase to organic phase. The effect of chloroform and dichloromethane as organic solvents over the metal chlorides extraction was investigated at 25 ± 0.1°C by using flame atomic absorption.
Keywords: Schiff bases; Hydrazones; Macrocyclic; Dihydrazide; Liquid-liquid extraction; Benzil
Schiff bases are widely studied and used in the fields of organic synthesis and metal ion complexation [1,2] for a number of reasons: their physiological and pharmacological activities [3-5] their use in ionselective electrodes [6-11] in the determination of heavy metals ions in environmental samples [12] and in the extraction of metals ions [13,14] and their many catalytic applications (e.g. for epoxidation of olefins, alkene cyclopropanation [15,16] trimethylsilylcyanation of ketones [17] asymmetric oxidation of methyl phenyl sulfide enantioselective epoxidation of silylenol [18] and ring-opening polymerization of lactide [19]).
Hydrazones are special group of compounds in the Schiff bases family. They are characterized by the presence of (C=N-N=C). the presence of two inter-linked nitrogen atoms was separated from imines, oximes, etc. hydrazone Schiff bases of acyl, aroyl and heteroacroyl compounds have additional donor sites like C=O. The additional donor sites make them more flexible and versatile. This versatility has made hydrazones good polydentate chelating agents that can form a variety of complexes with various transition and inner transition metals and have attracted the attention of many researchers.
Various hydrazones are obtained depending on the experimental conditions; which have application as biologically active compounds [20] and as analytical reagents [21]. As biologically active compounds, hydrazones find applications in the treatment of diseases such as anti-tumor [22] tuberculosis [22] leprosy and mental disorder [23]. Tuberculostatic activity is attributed to the formation of stable chelates with transition metals present in the cell. Thus many vital enzymatic reactions catalyzed by these transition metals cannot take place in the presence of hydrazones [24,25]. Hydrazones also act as herbicides, insecticides, nematocides, rodenticides and plant growth regulators.
In the context of the above applications we have reported here the synthesis and characterization of novel macrocyclic hydrazone Schiff bases. All these compounds (Scheme 1) have been characterized by elementa analyses, LC-MS, IR, 1H NMR, 13C NMR spectra data.
A Survey of the literature reveal that no work has been carried out on the synthesis of mcrocyclic hydrazone Schiff bases derived from pyridine-2,6-dicarbohydrazide and dicarbonyls. These Schiff bases have donor sites with the NON sequence and varied coordination abilities.
And in the present study, we synthesized hydrazone Schiff base (V) and used it as an organic chelating agent to extract some metal cations from their aqueous to another organic phase. For the application to analysis, solvent extraction conditions such as solution pH, the types of organic solvents, the concentration of Schiff base and the effect of aqueous to organic phase.
The preparation of new Schiff bases containing nitrogen, sulfur and oxygen donor atoms are shown in (Scheme 1). The structures of new compounds were characterized by a combination of elemental analyses, LC-MS, IR, 1H and 13C NMR spectral data. The preparation of the Schiff bases is illustrated in (Scheme 1).
IR spectra analysis
Compound (I): A strong band at 1620 and 1713 cm-1 in the IR spectrum of the Schiff base are assigned to υ(C=N) of azomethine and carbonyl υ(C=O) vibrations, respectively. An intense band at 3310 cm-1 is due to the -NH- vibrations of the hydrazine group and the band at 1068 cm-1 is assigned to hydrazinic υ(N-N) of the free ligand [26-30].
Compound (II): A strong band at 1621 cm-1 in the IR spectrum of the Schiff base is assigned to υ(C=N) of azomethine vibrations. An intense band at 3306 cm-1 is due to the –NH- vibrations of the hydrazine group and the band at 1068 cm-1 is assigned to hydrazinic υ(N-N) of the free ligand [26-30].
Compound (III): A strong band at 1621 cm-1 in the IR spectrum of the Schiff base are assigned to υ(C=N) of azomethine vibrations, respectively. An intense band at 3306 cm-1 is due to the -NH- vibrations of the hydrazine group and the band at 1068 cm-1 is assigned to hydrazinic υ(N-N) of the free ligand [26-30].
Compound (IV): A strong band at 1613 and 1690 cm-1 in the IR spectrum of the Schiff base are assigned to υ(C=N) of azomethine and carbonyl υ(C=O) vibrations, respectively. An intense band at 3306 cm-1 is due to the -NH- vibrations of the hydrazine group and the band at 1068 cm-1 is assigned to hydrazinic υ(N-N) of the free ligand [26-30].
Compound (V): A strong band at 1613 and 1674 cm-1 in the IR spectrum of the Schiff base are assigned to υ(C=N) of azomethine and carbonyl υ(C=O) vibrations, respectively. An intense band at 3306 cm-1 is due to the –NH- vibrations of the hydrazine group and the band at 1068 cm-1 is assigned to hydrazinic υ(N-N) of the free ligand [26-30].
1H-NMR spectral analysis
Compound (I): The 1H NMR spectrum (Figure 1) of the Schiff base (I), showed that the in the region 2.73 to 2.89 ppm were assigned to protons of methyl groups in two different environments [31]. The signals at 12.5 and 11.1 ppm were assigned to the protons of amide CONH and imine -CH=N groups respectively. Signals in the region 6.94–8.90 ppm were assigned to the aromatic protons.
Compound (II): The 1H NMR spectrum (Figure 2) of the Schiff base (II) dissolved in DMSO for increase of soluble, DMSO-d6, are depicted in (Figure 3). 1H NMR spectrum of the Schiff base (II) showed that in the signal at 5.2 ppm was assigned to protons of methyl groups [31]. The signals at 10.33 and 12.4 ppm were assigned to the protons of imine CH=N and thioamide NH groups respectively. Signals in the region 7.28-8.54 ppm were assigned to the aromatic protons.
Compound (III): The 1H NMR spectrum (Figure 3) of the Schiff base (III) dissolved in dimethyl-sulphoxide, DMSO-d6, are depicted in (Figure 4). 1H NMR spectrum of the Schiff base (III), showed that the signals at 1.87, 4.14 ppm were assigned to protons of methyl groups in two different environments [32]. The signals at 9 and 12.5 ppm were assigned to the protons of imine CH=N and thioamide NH groups respectively. Signals in the region 7.16-8.5 ppm were assigned to the aromatic protons.
Compound (IV): The 1H NMR spectrum of the Schiff base (IV), showed that the signals in the region 1.33-2.3 and 2.9 ppm were assigned to the protons of the methyl groups in two different [32]. The signals at 4.45 and 10.00 ppm were assigned to the protons of amino NH groups in two different environments, where one of them is deshielded to lower chemical shift than the other, this is due to the anisotropic effect of the neighboring keto (C=O) group. Signals in the region 7.88-8.42 ppm were assigned to the aromatic protons, where the protons are in different chemical environments. There is a very small peak at 12.1 ppm which might be due to the tautomerism of the ligand between N-H and C=O groups in the –NH(C=O)- moieties.
Compound (V): The 1H NMR spectrum of the Schiff base (V) in CDCl3 show signals at 11-11.5 ppm due to NH protons. Signals in the region δ 7.5-8.5 ppm due to aromatic protons [26-30].
Extraction of metal ions with Schiff base (V)
Effect of pH and solvents on the extraction of Cu(II) and Cr(III): The stability of a transition metal complex with a polydentate chelate ligand depends on a range of factors including: number and type of the donor atoms present and the number and size of the chelate rings formed on complexation [31]. In addition, the stability and selectivity of complexations strongly depend on the donor ability and dielectric constant of the solvent [33] and shape and size of the solvent molecules [34].
These results suggested that the amide phenyl and pyridine groups are the efficient group in the extraction and increasing a number of and nitrogen and oxygen donor increases the percentage of the extraction of the metal ions. It can be seen from figures that the solvent has an important effect upon the cation Extractability. These results may depend on dielectric constants of the solvents. The dielectric constants of dichloromethane and chloroform are 9.1 and 4.8 respectively. Dichloromethane having high dielectric constants is favored for the extraction of all the metal ions and there are similar results in literature [35]. On the other hand, the better solvation of the complexes by dichloromethane may be a valuable reason for better extraction. From the extraction data shown in figures, it is clear that the ligand which have N10O4, donor sets show that both of the cation-cavity size and the type of binding sites in the ring contribute to the ability of Cu(II) and Cr(III) ions binding.
(Figures 4 & 5) show the effect of pH on the extraction of Cu(II) and Cr(III) into chloroform and dichloromethane with Ligand. As shown in the figures the copper and chrome extraction are bigger as shown below within the pH range of 4.5-7.2, 5-8 respectively. Besides, Figures show that the ability of extraction is better in the case of dichloromethane solvent. Moreover transition metals were extracted in the order Cu (II)>Cr (III), which is in the same order as of decreasing ionic radius.
Composition of the extracted species: If only mononuclear species are extracted, under the condition in which chloride does not take part in the distribution equilibrium, the extraction process may be represented by Equation 1
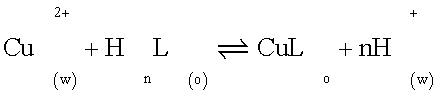 (1)
(1)
Where HnL (V) represents the extractant reagent and subscripts (w) and (o) denote the aqueous and organic phases, respectively. The extraction constant of the species CuL is given by Equation 2.
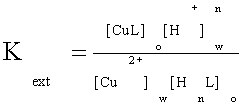 (2)
(2)
When CuL is the only extractable species and the metal is present in the aqueous phase predominantly as the cation Cu (II), the metal distribution ratio (D) and the extraction constant are related by
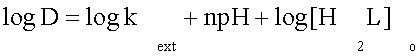 (3)
(3)
According to Equation (3) a plot of log D against pH at constant 4×10-4 M of [ HnL] will give straight line of slope is less from one and intercept log [HnL] + log Kext (Figure 6). These values represent the number of hydrogen ions (number <1) released during the formation of metal– ligand complex.
The results of the experiments at different concentrations of Schiff base (V) but constant chloride concentration revealed the 1:2(L:M) composition for Cu(II) and Cr(III) ions, when dichloromethane used as organic solvent (Figure 7).
Effect of aqueous to organic phase: Phase ratio (A ∕ O) is one of the factors that affect the extraction efficiency. The extraction efficiency, E% can be represented by [35]
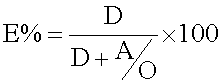 (4)
(4)
where D is the distribution ratio, A and O are the volumes of the aqueous and organic phases, respectively. Equation indicates that the extraction efficiency decrease with increasing A ∕ O ratio. (Figure 8) shown the effect of A ∕ O on percentage extraction which was satisfied by Equation 4.
The synthesized compounds act as hexadentate Schiff bases. In most cases these unsymmetrical compounds were obtained with yield more than 50% in some cases. Six imines (Schiff bases) were synthesized. Their structures were identified by spectroscopy methods. It is thought to be new and prepared for the first time. This was confirmed by a precise review of the scientific background concerning this category of compounds.
Furthermore liquid-liquid extraction of some transition metal ions (Cu(II) and Cr(III)) with ligand (V) have been examined. Schiff base (V) is good extractant for copper (II) and can be used for copper recovery.
The main following conclusions in extraction can be drawn out;
• Effect of pH: the extraction of Cu(II) and Cr(III) ions increased with increasing the pH of the aqueous medium.
• Concentration of the extraction: the extraction increased with the concentration of the polydentate Schiff base.
• The stoichiometry of the extracted species was determined by the conventional slope analysis method.
Finally, these results have established the feasibility of using simple and inexpensive extractants based on hydrazone Schiff bases to extract the heavy metal ions like Cu(II) by controlling their structure from aqueous medium.
Reagents and apparatus
All the used chemicals were purchased from Aldrich or Merck unless otherwise cited. The C, H and N were analyzed on a Carlo-Erba 1106 elemental analyzer. Mass spectra of the ligand were measured on a micro mass Quattro LC-MS Spectrometer. The IR spectra were obtained on Jusco 300 FT-IR Spectrometer with the samples in compressed KBr discs. 1H and 13C-NMR spectra of ligands in CDCl3 and C2D6SO solution were recorded on a Bruker 400MHz spectrometer and chemical shifts are indicated in ppm relative to tetramethylsilane. A Hitachi Model 180-80 Atomic absorptions Spectrometer (acetylene/ air flame) was used to determine the concentration of metal ions. A pH meter (Metrohm 691 pH Meter) was also used. All extractions were performed by using a mechanical flask agitator in 50cm3 stoppered glass flasks.
Synthesis
Synthesis of diethyl 2,6-pyridinedicarboxylate: 2,6-pyridine dicarboxylic acid (1.6 g, 8.2 mmol) in super dry ethanol (60 mL) containing 2-3 drops of concentrated H2SO4 (AR) was refluxed till it dissolved. Then, the reaction mixture was poured onto ice cold water, immediately a solid started separating from the clear solution. To this a solution of sodium bicarbonate was added till the effervescence seized. The ester thus obtained was filtered and washed with water for several times (mp 44-46°C) [36].
Synthesis of pyridine-2,6-dicarbohydrazide: A mixture of diethyl ester of 2,6-pyridine dicarpoxylic acid (2.22 g, 11.38 mmol) and hydrazine hydrate (98% 2 cc) in ethanol was refluxed for 5 hrs. 80% solvent was removed and water (20 mL) was added. Then the reaction mixture was kept for 8 hrs at -10°C. The white crystalline product (yield 80%) thus obtained was filtered, washed and dried [37].
Synthesis of pyridine-2,6-thiocarbohydrazide: To a suspension of pyridine-2,6-dicarbohydrazide (2.38 g, 12.2 mmol) in 20 mL of THF Lowessons reagent (4.95 g, 12.2 mmol) was added at room temperature, and the mixture was refluxed up to the formation of homogeneous solution (for 2 h). After removal of the solvent the residue was solved in 15mL CH2Cl2 and allowed to solidify. The crystals were dried and recrystallized from acetone. Pyridine-2,6-thiodicarbohydrazidewas obtained, yellow crystals, yield: 60%, mp 210-212°C.
Synthesis of 1,7-bis (5-bromo-2-formylphenyl)-1,4,7- trioxaheptane: To a stirred solution of 5-bromosalicylaldehyde (40 g, 200 mmol) and K2CO3 (13.8 g, 100 mmol) in DMF(100 mL), was added drop wise 1-chloro-2-(2-chloroethoxy)ethane (14.3 g, 100 mmol) in DMF (40 mL). The reaction was continued for 4 h at 150-155°C and then for 4 hrs at room temperature. Then, 200 mL distilled water was added and the mixture was kept in refrigerator. After 1 h, the precipitate was filtered and washed with 500 ml water. It was dried in air and recrystalized from EtOH and filtered under vacuum. Yield: 85%, mp 180-182°C.
Synthesis of α,α’-bis(5-bromo-2-carboxyaldehyde phenoxy) xylene: To a stirred solution of 5-bromosalicylaldehyde (40 g, 200 mmol) and K2CO3 (13.8 g, 100 mmol) in DMF (100 mL), was added dropwise α,α’-dibromo-p-xylene (17.5 g, 100 mmol) in DMF (40 mL). The reaction was continued for 4 hrs at 150-155ºC and then for 4 hrs at room temperature. Then, 200 mL distilled water was added and the mixture was kept in refrigerator. After 1 hr, the precipitate was filtered and washed with 500 mL water. It was dried in air and recrystalized from EtOH and filtered under vacuum. Yield: 80%, mp 228-230°C.
Synthesis of 1,5-bis(5- bromo-2-formylphenyl) pentane: To a stirred solution of 5-bromosalicylaldehyde (40 g, 200 mmol) and K2CO3 (13.8 g, 100 mmol) in DMF (100 mL), was added dropwise 1,5-dibromopentane (22.8 g, 100 mmol) in DMF (40 mL). The reaction was continued for 4 hrs at 150-155°C and then for 4 h at room temperature. Then, 200 mL distilled water was added and the mixture was kept in refrigerator. After 1 h, the precipitate was filtered and washed with 500 mL water. It was dried in air and recrystalized from EtOH and filtered under vacuum. Yield: 75%, mp 169-171.
1,2,8,9-tetraza-4,6; 11,12; 20,21-tribenzo–3,7-dicarbonyl-13, 16, 19- trioxa- cyclodocosane-1,9-diene (I): The macrocyclic Schiff base (I) was prepared by dropwise addition of a solution of the pyridine- 2,6-dicarbohydrazide ( 0.386 g, 2 mmol) in DMF (40 mL) to a stirred solution of 1,7-bis(5- Bromo-2-formylphenyl) -1,4,7-trioxaheptane (i) (0.94 g, 2 mmol) in DMF (60 mL) containing a few drops of concentrated HCl. The reaction mixture was heated to reflux for 5 hrs, where yellow precipitate was formed after cooling. On cooling, 200 ml distilled water was added and the mixture was kept in a refrigerator. After 2 hrs, the precipitate was filtered and washed with 200 mL water. The solid obtained was collected and recrystallized from mixture DMFH 2O as yellow crystals. A yellow colored precipitate was washed with water, ethanol, CHCl3 and diethyl ether, respectively. Then dried in air. Yield: 60%. mp 288-290. Anal. Calc. for C25H21Br2N5O5: C, 47.69; H, 3.33; N, 11.1. Found: C, 47.65; H, 3.4; N, 11.0%, Mass spectrum (LCMS): m/z=629 ([C25H21Br2N4O5]). IR (cm-1) υ C=O 1713; υ C=N 1620; υ N–N 1068. 1H NMR ((CD3)2SO), 400 MHz): 2.73 (t, 4H), 2.89 (t, 4H), 6.94-8.90 (d, m, 9H), 11.1 (s, -N=CH-), 12.5 (s, -CONH-) ppm. 13C NMR ((CD3)2SO), 75 MHz): d (ppm) spectrum of Schiff base (I) indicated new resonances are: 31.22, 36.2 (-OCH2CH2O-), 111.16, 119.21, 122.14, 126.24, 130.17, 134.44 (Phenyl-C), 140.55, 146.82, 149.44 (all pyridine-C), 156.84 (CH=N), 160,162.78 (CO-NH) (Figure 1).
1,2,8,9-tetraza-4,6; 11,12; 15,18; 21,22-tetrabenzo-3,7- dithiocarbonyl-13,20-dioxa-cyclotricosane -1,9-diene (II): The macrocyclic Schiff base (II) was prepared by dropwise addition of a solution of the pyridine-2,6-dithiocarbohydrazide (0.454 g, 2 mmol) in DMF (40 mL) to a stirred solution of α,α’-bis(5-bromo-2- carboxyaldehyde phenoxy)-1.4-xylene (ii) (1.0 g, 2 mmol) in DMF (60 mL) containing a few drops of concentrated HCl for about 1-2 hrs on a water bath. After the addition was completed the reaction was heated to reflux for 5 hrs, where yellow precipitate was formed after cooling. On cooling, 200 mL distilled water was added and the mixture kept in a refrigerator. On cooling, 200 mL distilled water was added and the mixture was kept in a refrigerator. After 2 hrs, the precipitate was filtered and washed with 200 mL water. The solid obtained was collected and recrystallized from mixture DMF-H2O as yellow crystals. A yellow colored precipitate was washed with water, ethanol, CHCl3 and diethyl ether, respectively. Then dried in air. Yield: 60%, mp >280°C Anal. Calc. for C29H21Br2N5O2S2: C, 50.21; H, 3.03; N, 10.1%. Found: C, 50.22; H, 3.1; N, 10.12 %. Mass spectrum (LC-MS): m/ z=693 ([C29H21Br2N5O2S2]). IR (cm-1) υ C=N 1621; υ N–N 1068. 1H NMR ((CD3)2SO, 400 MHz): 5.4 (s, 4H), 7.28 - 8.54 (m, 13H), 10.3 (s, -N=CH-), 12.3 (s, -CSNH-) ppm. 13C NMR ((CD3)2SO, 75 MHz): d (ppm) Schiff base (II) indicated new resonances are: 70.42 (-CH2 -O-) 113.05, 113.22, 117.37, 126.53, 128.92, 130.40, 130.50, 136.45, 137.08 (all phenyl-C) 138.87, 157.09, 160.22 (all pyridine-C), 162.78 (C=N) 188.59 (CS-NH) (Figure 2).
1,2,8,9-tetraza-4,6; 11,12; 20,21-tribenzo -3,7-dithiocarbonyl -13,19-dioxa-cyclodocosane-1,9-diene (III): The macrocyclic Schiff base (III) was prepared by dropwise addition of a solution of the pyridine-2,6-dithiocarbohydrazide ( 0.454 g, 2 mmol) in DMF (40 mL) to a stirred solution of 1,5-bis (5-bromo-2-formylphenyl) pentane (iii) (0.94 g, 2 mmol) in DMF (60 mL) containing a few drops of concentrated HCl for about 4-5 hrs on a water bath. After the addition was completed, the reaction was heated to reflux for 5 hrs, where yellow precipitate was formed after cooling. On cooling, 200 ml distilled water was added and the mixture was kept in a refrigerator. After 2 h, the precipitate was filtered and washed with 200 ml water. The solid obtained was collected and recrystallized from mixture DMF-H2O as yellow crystals. A yellow colored precipitate was washed with water, ethanol, CHCl3 and diethyl ether, respectively. Then dried in air. Yield: 60%, mp> 280°C Anal. Calc. for C26H23Br2N5O2S2: C, 47.34; H, 3.49; N, 10.6. Found: C, 47.33; H, 3.5; N, 10.6%. Mass spectrum (LC-MS): m/z=659 ([C26H23Br2N5O2S2]). IR (cm-1) υ C=N 1621; υ N–N 1068. 1H NMR ((CD3)2SO, 400 MHz): 1.87 (m, 6H) 4.14 (t, 4H), 7.12-8.39 (m, 13H), 8.95 (s, -N=CH-), 12.5 (s, -CSNH-) ppm. 13C NMR ((CD3)2SO, 75 MHz): d (ppm) Schiff base (III) indicated new resonances are; 24.81, 30.51 (-CH2CH2 -), 69.31 (-CH2O-) 112.77, 115.30, 124.41, 125.96, 127.92, 134.46 (Phenyl-C), 140.63, 143.51, 148.38 (all pyridine-C), 157.9 (CH=N), 160 (CSNH) (Figure 3).
Macrocyclic 2,6-pyridine dicarpoxylic acid hydrazone Schiff base (IV): The hot ethanolic solution (20 mL), of pyridine-2,6- dicarbohydrazide ( 3.9 g, 20 mmol), and a hot ethanolic solution (20 mL), of acetylacetone ( 2 g, 20 mmol) were mixed slowly with constant stirring. This mixture was refluxed at ~75°C for 9 h in the presence of few drops of concentrated hydrochloric acid (pH ~ 3). On cooling cream colored precipitate is separated out, which was filtered, washed with cold EtOH, and dried under vacuum over P4O10. Yield 68%, mp >280°C. Anal. Calc. for C24H26N10O4: C, 55.6; H, 5.02; N, 27.02. Found: C, 55.56; H, 4.98; N, 27.01%. Mass spectrum (LC-MS): m/z=518 ([C24H26N10O4]). IR (cm-1) υ C=O 1690; υ C=N 1613; υ N–N 1068. 1H NMR ((CDCl3), 400 MHz): 7.28 (s, H), 1.33 (s, 6H), 2.9 (s, 4H) 4.45 (s, -CONH-) 7.88-8.42 (m, 13H) ppm. 13C NMR ((CDCl3, 75 MHz): d (ppm) Schiff base (IV) indicated new resonances are; 31.46, 36.53(- CH3) 62.26 (-CH2-), 127.88, 138.65 (all pyridine-C), 148.61(CH=N), 162.58(CO-NH).
Macrocyclic 2,6-pyridine dicarpoxylic acid hydrazone Schiff base (V): Hot ethanolic solution (20 mL) of benzil (4.2 g, 20 mol), and a hot ethanolic solution (20 mL) of pyridine-2,6-dicarbohydrazide (3.9 g, 20 mmol) were mixed slowly with constant stirring. This mixture was refluxed at 80°C for 6 hrs in the presence of few drops of concentrated hydrochloric acid. On cooling, white colored precipitate was formed. It was filtered, washed with cold EtOH, and dried under vacuum over P4O10. Yield %60, m.p 88-90°C. Anal. Calc. for C42H30O4N10: C, 68.29; H, 4.06; N, 18.97. Found: C, 68.24; H, 4.1; N, 18.96%. Mass spectrum (LC-MS): m/z= 738 ([C42H30N10O4]). IR (cm-1) υ C=O 1674; υ C=N 1613; υ N–N 1068. 1H NMR (CDCl3, 400 MHz): 7.28 (s, 6H), 7.5-8.5 (m, 26H), 11 (s, -CONH-) ppm. 13C NMR (CDCl3, 75 MHz): d (ppm) Schiff base (V) indicated new resonances are; 125.972, 126.08, 128.43, 129.042, 129.62, 129.67, 129.74, 129.92, 131.004, 131.05, 132.99, 133.42, 139.6 (Phenyl-C), 147.47, 147.828, 152.98 (all pyridine-C), 158.84, 163 (-C=N-) 190, 195.59 (-CONH-)
Aqueous solutions containing 1.5 x 10-3 M Metal chloride in appropriate buffer were equilibrated with equal volumes of the chloroform and dichloromethane solutions of the ligand 4 x 10-4 M by shaking in a mechanical shaker at 25ºC. Optimum equilibration time was determined for this system. In most cases distribution equilibrium was attained in less than 120 min and a shaking time of 120 min. The ionic strength of the aqueous phase was 0.1 M KCl in all experiments except those in which the effect of ionic strength was studied. After agitation, the solutions were allowed to stand for 120 min. The Cu2+ and Cr3+ concentrations of the aqueous phase were determined by FAAS, and that of the organic phase from the difference by considering the mass balance. The pH of aqueous phase was recorded as equilibrium pH.
We are grateful to Department of Chemistry, Faculty of Science, Damascus University, Syria for the support of this research.