Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2017) Volume 7, Issue 3
Cisplatin is a commonly used anticancer drug which is the first generation of platinum-based anticancer drugs developed. The cis configuration enables the binding of the coordination complex to one or two DNA strand (s) and thereby crosslinking the DNA strands triggering the cells to die in a programmed manner. It is used to treat various types of cancers such as small cell lung cancer, ovarian cancer, bladder cancer, cervical cancer, and germ cell tumours. When administered into blood, cisplatin reacts with thiol containing proteins present in blood plasma thus reducing its bioavailability, increasing cytotoxicity and it is associated with numerous side effects including nephrotoxicity, neurotoxicity, nausea, vomiting and ototoxicity. In order to increase the bioavailability and to reduce dosage and cytotoxic effects to healthy cells thermodynamically unstable vaterite form of Porous Calcium Carbonate Nanoparticles are synthesized using the soft molecular template approach to synthesize them from aqueous solution. Hydrogen bonded networks of ethylene glycol and water are formed by mixing appropriate quantities of the two liquids to have spherical templates. Cisplatin is encapsulated in porous nanoparticles of the vaterite form of CaCO3 and studied the release kinetics of the drug in the solutions at different pH values. It is found that the drug is released slowly and steadily in the pH values of cancerous cells (pH range 5.0–6.0) but not in the pH ranges exhibited by healthy cells (pH range 7.0-8.0). Here, the synthesis and stabilization of nanoparticles of vaterite as well as their characterization, encapsulation of cisplatin and the slow minimum amount of constant release kinetics of the drug at various pH values are described. This is a way forward for safe and convenient chemotherapeutic route to various cancers.
Graphical Abstract
Keywords: Vaterite polymorph; Anticancer drug; Cisplatin; Encapsulation; pH triggered
Calcium carbonate particles have prevalent technological applications in building, paper, pharmaceutical, textile, rubber, plastic, paint, cosmetic, toothpaste, glove, cosmetic, food and beverage, sealing wax, ink, plastic film manufacturing and many other manufacturing industries. In medicine and healthcare products, calcium carbonate is used as inexpensive dietary calcium supplement, phosphate binder for the treatment of hyper phosphatemia in patients with renal failure and also as an inert filler of tablets and pharmaceuticals [1,2]. In all these practical applications, if macro or micro particles that are currently used could be replaced by respective nanoparticles then the material requirement can be drastically reduced since nanoparticles have extremely large surface area to volume ratio. For instance 1 kg of 1 μm3 particles has the same surface area as 1 mg of 1 nm3 particles. Hence, in terms of surface area matching the material requirement can be reduced million-fold if 1 μm3 particles could be replaced by 1 nm3 particles, at these extreme ends.
Different polymorphs of Calcium Carbonate (CaCO3) exist in various amorphous forms, Amorphous Calcium Carbonate (ACC), two hydrated forms; Calcium Carbonate Monohydrate (MCC) and calcium carbonate hexahydrate (ikaite) and three anhydrous polymorphs, namely, vaterite, aragonite, and calcite. Vaterite is the least stable polymorph of calcium carbonate [3]. Its synthesis requires special techniques such as use a very specific polymers like polyaniline [4] or nanocomposites such as nanocomposites of alkyd resin/ polyaniline/vaterite [5] for stabilization. Vaterite has the same chemical composition as calcite (rhombohedral) and aragonite (orthorhombic), but with a different crystal structure in terms of symmetry, orientation of CO32− ions, and coordination environment of Ca ions. It is spherical in shape and this shape has the advantage that it does not have sharp edges. Therefore, when vaterite is used as drug carriers they do not damage biological cells by mechanical actions such as cutting by extruded sharp edges. The formation of different polymorphs of CaCO3 is highly affected by precipitation conditions. Among them, Temperature, pH and additives (e.g. phosphate) are considered to be two governing factors regulating polymorphism during calcium carbonate precipitation. Vaterite is a preferable polymorph at medium temperature ranging from 25 to 45 °C. Abundance of different vaterite morphologies (spherical, star-like, cubes like or irregular) in response to temperature can be explained with the help of the theory of nuclei agglomeration and growth [6].
Almost all of the current synthetic methods developed to prepare calcium carbonate nanoparticles usually give either amorphous calcium carbonate or calcite since these two forms are the thermodynamically most stable forms of calcium carbonate. However, as far as some specific applications such as the use as drug carriers, are concerned, calcite has some drawbacks; the major one being its rhombohedral shape which has sharp edges. In this research, we used a water-Ethylene glycol soft molecular template to prepare porous nanoparticles of vaterite which has spherical shape of particles. The well characterized vaterite nanoparticles were used to encapsulate the anticancer drug cisplatin (Figure 1).
Cisplatin also known as cisplatinum, platamin, neoplatin [7], with the chemical formula of cis-diamminedichloridoplatinum (II) (CDDP) is a first generation of platinum coordination compound-based anticancer chemotherapeutic drugs that is used to treat small cell lung cancer, ovarian cancer, lymphomas, bladder cancer, cervical cancer and germ cell tumours. Cisplatin is a yellow to orange crystalline powder. Cisplatin is a heavy metal complex comprising a central atom of platinum enclosed by two chloride atoms and two ammonia molecules in the cis position (Figure 2). It is soluble in water or saline at 1 mg/mL and in DMF (Dimethylformamide) at 24 mg/ml. Melting point of cisplatin is 207°C. It is found that cisplatin is predominantly effective against testicular cancer with a cure rate of 12-85% [8]. The cis configuration enables the binding of the coordination complex to two DNA strands and thereby crosslinking the DNA strands which triggers the cells to die in a programmed manner. Furthermore cisplatin complex transfers through cell membranes in a unionised form and this is attained in the comparatively high chloride concentration in the plasma. Intra cellularly the concentration of chloride ions is lesser than in the plasma and the chloride ligands on the cisplatin complex are displaced by water molecules. The consequence is the creation of positively charged platinum complexes that are toxic to cells. The cisplatin molecule binds with the DNA molecule at the guanine bases and thus constrains DNA, RNA and protein synthesis. The cisplatin anticancer drug forms intra-strand and inter-strand cross links in the DNA molecule and performs to correlate well with the cytotoxicity of the anticancer drug. The tumour cells accumulate an overload of mutations which lead eventually to the cell’s death. Cisplatin also has radio sensitizing, immunosuppressive, and antimicrobial properties as well. The exact mechanism of action of cisplatin is not yet revealed [9,10]. However, cisplatin is associated with numerous side effects which include nephrotoxicity, neurotoxicity, nausea and vomiting, ototoxicity (hearing loss), electrolyte disturbance and haemolytic anaemia. The successful encapsulation of cisplatin in hollow vaterite nanoparticles described here is the first step in targeted delivery of cisplatin to cancerous cells which we are currently pursuing.
Sustained release or controlled release drug therapy systems that are designed to accomplish a persistent therapeutic effect by constantly releasing medication over an extended period of time after administration of single dose of the drug. The objective in designing sustained delivery systems is to minimise the frequency of the dosing or to upsurge effectiveness of the drug by localization at the site of action, decreasing the dose required or supplying constant drug delivery. Accordingly, in sustained release dosage system it releases one or more drugs unremittingly in a prearranged pattern for a fixed period of time, either systemically or to a specifically targeted organ in the body. Sustained release dosage forms afford a less dosage frequency, a better control of plasma drug levels, less side effects, increased efficacy and persistent delivery [11]. In controlled release systems therapeutic agents’ maybe automatically delivered at predetermined rates over a stretched period of time [12]. There are certain concerns for the formation of Sustained drug release formulation. Basically, if the active compound has a long half-life (over 6 h), it can be sustained on its own.
If the absorption of the active compound involves an active transport as the transport method through the body, the development of a time-release product may be challenging. In addition to passive diffusion across the cell membrane, other transport mechanisms (endocytosis and some active or facilitated transport methods) are currently available in the uptake of platinum-based drugs. Lastly, if the active compound has a short half-life, it would require a large amount to maintain a lengthy effective dose [13]. The advantages of Sustained drug delivery system are the frequency of drug administration is abridged, Drug administration can be made more convenient, Better control of drug absorption can be achieved, since the high blood level peak that may be observed after administration in an extended action form, The total amount of drug administration can be decreased, Progress efficacy in treatment, Cure or control condition more promptly.
Cancer is the name given to a group of related diseases. In all types of cancer, some of the body’s cells commence to divide without ending and spread into neighbouring tissues. Cancer can start virtually anywhere in the human body, which is has trillions of cells. Generally, human cells grow and divide to produce novel cells as the body requires them. When cells grow old or become damaged, they die, and new cells replace. Once cancer grows, however, this methodical process breaks down [14]. Since cells become more and more irregular, old or damaged cells survive when they should die, and new cells form when they are not required. These extra cells can divide without discontinuing and may form growths called tumours. Many of cancers process solid tumours, which are masses of tissue in the body. Cancers of the blood, such as leukaemia, commonly do not form solid tumours [15]. Cancerous tumours are malignant, which means they can spread into, or conquer, nearby tissues. In addition, once these tumours grow, some cancer cells can break off and transport to distant places in the body through the bloodstream or the lymph system and produce novel tumours differs from the original tumour. Disparate malignant tumours, benign tumours do not spread into nearby tissues. Benign tumours can sometimes be fairly huge. When removed, they usually don’t grow back, whereas malignant tumours sometimes grow. But benign brain tumours can be life threatening.
All the chemicals except cisplatin were purchased from Sigma Aldrich and they were of analytical grade and used without further purification. Cisplatin injection bottles containing 1% cisplatin in saline water was purchased from Sri Lanka Pharmacy, Kandy, Sri Lanka.
Porous CaCO3 nanoparticles were synthesized by the reaction between Ca(CH3COO)2 and NaHCO3 in a solvent mixture of H2O and Ethylene Glycol (EG). Solution A is a mixture of Ca(CH3COO)2 (25.0 mL, 0.50 M), H2O (10.0 mL) and EG (25.0 mL). Solution B is a mixture of NaHCO3 (25.0 mL, 0.50 M), H2O (10.0 mL) and EG (25.0 mL). Then, the solution B was added to solution a slowly in drop-wise manner under stirring, using a magnetic stirrer, at 250 rpm speed, at room temperature. Stirring was continued for further 24 h and the resultant suspension was centrifuged. The solid mass obtained was washed first with ethanol and then subsequently with distilled water and dried at 100ºC for 4 h in a vacuum oven. The CaCO3 particles thus obtained were characterized by Laser Light Scattering based Particle Size Analysis (CILAS Particle Size Analyser NANO DS), XRD (Siemens D5000 X-ray powder diffractometer), XRF, FT-IR (Shimadzu IR-Prestige 21 Instrument with the KBr pellet method and SEM (Environmental SEM with EXAS Facilities) studies. The surface area of the Porous CaCO3 nanoparticles was determined by the novel BET instrument constructed in our research group. This instrument is similar to that of commercially available BET systems for monolayer gas absorption. But our home-made one has a more accurate Pressure Sensor Detector. Specific surface area measurements are carried out using standard nitrogen gas adsorption method and are calculated comparing the signal obtained for the sample with that of a standard of known surface area. Samples are cooled using liquid N2 at -193°C and partial pressure ratio of inert gases is He: N2.3:1. The standards used are TiO2 nanoparticles of specific surface area 600 m2 g-1, 1500 m2 g-1, carbon black with of specific surface area 1200 m2 g-1 and 1500 m2 g-1. In the encapsulation of cisplatin drug into the hollow CaCO3 nanoparticle, 0.50 g of CaCO3 nanoparticles were dispersed in a 100 mL of cisplatin injection solution and kept stirring for 24 h to facilitate cisplatin encapsulation. The product was subsequently separated by centrifuging followed by suction filtration and washed several times with distilled water. The encapsulation of cisplatin was confirmed by the FT-IR and XRF analyses of the products obtained. 0.8 g of anticancer drug cisplatin encapsulated product was put into 14000 cut off cellulose dialysis tubes and were placed in beakers containing buffer solutions with pH values of 4.0, 5.0, 6.0, 7.0 and 8.0. Then the five different pH buffer solution systems were placed on thermostatic shaker kept at 37°C 100 rpm. 8 ml of supernatant solution was withdrawn at 1h time intervals for 24 h while equal volumes of fresh buffer solutions were added. Platinum content of the buffer supernatant was determined by using Atomic Emission Spectroscopy. Calibration curve was developed in order to find the concentrations of platinum which is released by the encapsulated products using 1ppb standard Chloroplatinic acid solution.
As shown in Figure 3, particle size analysis shows that the colloidal solution comprises particles in the nano-range between 20 nm to 80 nm with average particle size of 36.9 nm in 90% confidence interval. It is thought-provoking to note that the wet chemical synthesis of porous CaCO3 nanoparticles gives such a narrow distribution of non-aggregated nanoparticles of calcium carbonate which is not usually straightforward. Hence, the soft molecular template of hydrogen bonded network of supplementary ethylene glycol molecules and lesser water molecules form cavities of this size.
Ethylene glycol-water mixture forms hydrogen-bonded cage-like arrangements in which relative proportions of EG and water molecules depend upon the molar ratio of EG: water used. In pure ethylene glycol only intermolecular hydrogen bonding exists and that there are no intra-molecular hydrogen bonding formed [16,17]. As such, hydrogen bonded cage-like structures are available in pure ethylene glycol. In EG: water mixtures the relative ratio of EG: water in inter-molecular hydrogen bonded structures and chemical bond arrangements depend on the EG: water molar ratio used. Figures 4a and b show the computationally optimised EG and water cluster structures bonded with H bonds for possible two different molecular ratios. G is miscible with water in all proportions and can form three dimensional networks of hydrogen bonded molecules similar in nature to those of water [17,18] owing to two proton donor hydroxyl groups and two oxygen atoms plays the role of accepting protons in hydrogen bonds. Amount of hydroxyl groups in the reaction medium is of particular significance as far as the particle size is concerned. Molecules of EG in the pure liquid phase participate in the intermolecular hydrogen bonding, although the intramolecular bonds are not observed. In water-poor regions EG-water interactions are favored over water–water ones [19]. The molecules of water are doubly-bonded to two dissimilar molecules of EG and this cooperative interaction is stronger than that in bulk water. An increase in the water content weakens the average strength of the hydrogen bonding of water but has a small effect on the structure of liquid EG. The size of these clusters depends on the concentration of EG, the smaller concentration of EG, the larger the size of water clusters. It is vital to note that high temperature synthesis in water/ ethylene glycol medium does not seem to be an option for getting the nano-sized crystals of vaterite. What happens here is that the influence of temperature on the particle size is hardly noticeably unlike the clear effect of hydroxyl groups [20].
Polar alcohol groups of EG molecules also possess a high cohesive energy for oppositely charged Ca2+ ions. Strong association of calcium ions and alcohol groups locally increases super-saturation so that nucleation occurs at a much faster rate near these bound ions than in bulk. Once all the sites are occupied by crystal nuclei, it becomes energetically favourable for the remaining ions to associate with these growing particles instead of developing new nuclei. Hence, the more ions are present in solution, the more material will be spent for particle growth because of the constant number of nuclei in the system. CaCO3 is a “reverse soluble” compound that becomes less soluble at higher temperatures due to decreased solubility of carbon dioxide in water [21,22]. besides, as the Equations 1 and 2 suggest, the nucleation rate increases with temperature. These two factors allow us to propose benefits of higher temperatures for formation of smaller vaterite particles and preclusion of the resultant recrystallization process to steady polymorphs. Calcium carbonate particles obtained via mixing of Ca(CH3COO)2 and NaHCO3 salts were fairly unvarying and non-aggregated spherical stuctures whose diameter particularly depended on salt concentration [23]. According to the classical theory of nucleation and growth [24], a higher concentration of salt rises supersaturation process, which results in a considerable nucleation rate as proposed by Equation 1 and 2. Supersaturation is defined as S in the Equation 3. A higher nucleation rate consequently results in a smaller particle size at the same total mass of precipitate.
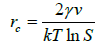 (1)
(1)
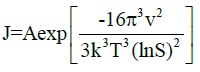 (2)
(2)
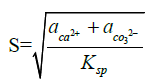 (3)
(3)
Where ‘rc’ is the radius of critical nucleus, ‘γ’ is the interfacial tension, ‘ν’ is the volume of a molecule inside the nucleus, ‘k’ is the Boltzmann constant, ‘T’ is absolute temperature, ‘S’ is the super-saturation, ‘J’ is the nucleation rate, ‘Ksp’ is the thermodynamic solubility product for vaterite polymorph, and ‘a’ is the ionic activity coefficient.
The SEM images of such porous nanoparticles of vaterite formed before and after the anticancer drug encapsulation are shown in Figures 5a and 5b respectively. The SEM images obviously show that the sample contains spherical particles of nano-range but bunched into aggregated particles. This clustering might have occurred during deposition of particles onto glass surface when SEM analysis was being done. As is evident from particle size determination of the suspension, discrete particles are present in the suspension. The morphology of the particles indicates a good porous nature. The BET surface area analysis gives 1500 m2/g indicating that the particles have a huge surface area conceivably due to their porous nature of the nanoparticle. Such high specific surface area for the nanoparticles must have originated due to the exposure of the most of the atoms/ions in the interior of the particles due to very high porosity of the particles. These pores must have been formed during the heat treatment when the entrapped soft template, EG and water molecules within the nanoparticles are removed. This porous nature of the nanoparticles is very much significant and important in entrapping the anticancer drug Cisplatin. The SEM images are quite comparable to those obtained for vaterite nanoparticles without cisplatin encapsulation. This shows that vaterite form of calcium carbonate is well preserved even after the cisplatin encapsulation.
IR spectroscopy is expedient and useful to distinguish the dissimilar crystal phases of calcium carbonates. The molecular structures of calcium carbonates, comprising two ions of calcium and carbonate, are simple and the vibration of carbonate ion in different calcium carbonate structures could be distinguished using IR spectra.
The absorption bands of carbonate are divided into four areas where symmetric stretching is at 1080 cm-1, out of- plane bending is at 870 cm-1, doubly degenerates planar asymmetric stretching is available at 1400 cm-1 and doubly degenerate planar bending is at 700 cm-1 [25,26]. The bulk vaterite is characterized by its characteristic IR absorption bands centred at 877, 745.8, 1084, 1427 and a split peak at 1440 and 1490 cm-1 [27]. As evident from the FT-IR spectrum, provided in Figure 6, absorption band at 887 cm-1 has been shifted to 882 cm−1 and that at 1084 cm-1 has been shifted to 1087 cm-1 in nanoparticles which are close to vaterite absorption bands than those of other forms. Such a small shift in IR absorption band maximum is expected in nanoparticles. The bands at 745.8, 1427 and a split peak at 1440 and 1490 cm-1 also help to confirm the presence of the vaterite polymorph of CaCO3. No absorption bands at 2522, 873, 854, 712, 700 cm-1 and 848, 714 cm-1 corresponding to aragonite and calcite respectively, are present in the spectrum. Since vaterite is thermodynamically most unstable form of CaCO3, it is very stimulating that our procedure produces and stabilizes this unstable vaterite form rather than forming stable calcite polymorph form. The use of molecular soft templates formed by hydrogen-bonded EG and water molecules cluster structure direct the vaterite formation allowing crystallizing in spherical manner.
The FT-IR spectrum of the encapsulated product obtained (Figure 6b) clearly show the presence of N-H antisymmetric and symmetric stretching vibration 3373 cm-1 and 3597 cm-1, N-H wagging together with CO32− vibrations, deformation vibrations (scissoring) 1650 cm-1, 1590 cm-1 and wagging oscillation broad transmittance band 800 cm-1. It also contains all the bands that are reported for hollow nanoparticles of CaCO3. In the FTIR spectrum, the absorption bands at 871 cm-1 show the presence of vaterite polymorph. As such, the FT-IR data suggest the encapsulation of cisplatin in hollow CaCO3. Interestingly, in the encapsulated product the intensity of the band at 1087 cm-1 which is due to stretching mode of carbonate ion vibration has been significantly increased compared to that at 882 cm-1 which responsible for the bending mode of carbonate ion vibration. Cisplatin has two NH2 groups which can form H-bonding with carbonate O atoms. When these H-bonds are formed the bending of carbonate ion is made difficult compared to its stretching. Therefore, this change in relative intensities of carbonate stretching and bending vibrations also give an indication to the presence of cisplatin within vaterite nanoparticles.
We have further characterized the calcium carbonate nanoparticles prepared in this work using X-ray diffractometry and the X-Ray Diffractogram obtained is included in Figure 7a.
As can be seen from Figure 7a, the sample contains essentially vaterite as the major phase. The main characteristic peaks of vaterite at 2θ of 20.88°, 24.94°, 26.99°, 32.78°, 38.73°, 39.29°, 40.56°, 41.66°, 42.57°, 43.74°, 48.95°, 49.92°, 50.93°, 52.19°, 55.65° correspond to the (004), (110), (112), (114), (211), (205), (116), (213), (008), (300), (304), (118), (220), (208) and (224) crystallographic planes, respectively can be clearly observed [28]. A very trivial fraction of calcite polymorph particles can also be observed though the percentage abundance is insignificant compared to that of vaterite nanoparticles. Application of the Debye- Scherrer Equation to the major XRD peak gives the crystallite size to be 28.0 nm. This is very close to the average particle size obtained by the laser light scattering based particle size analysis. This might be the reason why both FT-IR and SEM techniques do not detect the polymorph of calcite. The presence of a minute fraction of calcite is not astounding since it is the thermodynamically most stable phase of calcium carbonate and at the same time presence of these nanoparticles has no adverse effect on the encapsulation of cisplatin.
The X-Ray Diffractogramme of the cisplatin encapsulated vaterite nanoparticles is shown in Figure 7b. It also comprises vaterite as the major component with minute fractions of calcite and sodium chloride. The presence of cisplatin cannot be determined from the XRD since cisplatin is present in molecular form within the restricted environment of pores of vaterite nanoparticles in non-crystalline form. However, FTIR and XRF studies complement this showing the presence of cisplatin in encapsulated nanoparticles. The encapsulation has not affected the crystallinity and crystallite size of vaterite nanoparticles. If we consider the 2θ values and crystallographic planes, it is obvious that there is no significant difference between pure vaterite CaCO3 nanoparticles and cisplatin encapsulated CaCO3 nanoparticles. The crystallite size calculated using the major XRD peak is 29.4 nm which is close to that without cisplatin encapsulation. Slight expansion of the crystallite size is expected when cisplatin is occupying otherwise empty spaces. Hence cisplatin molecules occupy the free spaces of porous nanoparticles of vaterite without significantly changing the particle dimensions.
XRF of the CaCO3 nanoparticles synthesized detects only Ca in our sample since our XRF machine is incapable of detecting elements with atomic number less than 13. Hence it shows that the product formed is free of impurities comprising elements with atomic number higher than 13. The XRF spectrum of the cisplatin encapsulated CaCO3 product shown in Figure 8 clearly elicits the presence of Ca, Pt and Cl with the Pt: Cl atomic ratio of 1:2.5 showing that cisplatin maintains its identity in the confined environment also. Ideally, the ratio should be 1:2 but the presence of excess chloride in saline solution would encapsulate free chloride ions also thus increasing the chloride content. XRF data suggest the encapsulation of cisplatin in Porous CaCO3 nanoparticles.
The EDAX analysis of SEM images of both vaterite nanoparticles and cisplatin encapsulated vaterite nanoparticles show the presence of Ca, C and O. It is captivating to note that the EDAX analysis does not show Pt, Cl or N in the cisplatin encapsulated vaterite nanoparticles though XRF obviously shows the presence of Pt and Cl and FT-IR gives evidence to the presence of N. Both XRF and FT-IR are bulk analytical techniques that measure the composition of the bulk sample. However, SEM analysis is a surface analytical technique detecting 5 nm widths from the surface and hence EDAX detects the elements present close to the surface of the vaterite nanoparticles. Since the elements of cisplatin cannot be detected within 5 nm depth of the surface and bulk techniques detect them it can be concluded that cisplatin is present deep inside the particles at least deeper than 5 nm width from the surface. This is a clear evidence to confirm that cisplatin is encapsulated in the centres of nanoparticles.
Release kinetics studies
CaCO3 is a stable material under basic conditions. At the same time it is sensitive to acidic pH medium where it tends to be readily soluble. Yet it is also slightly soluble at all pH values below 7.
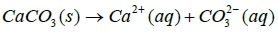 (4)
(4)
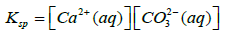 (5)
(5)
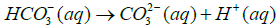 (6)
(6)
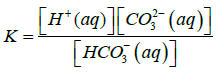 (7)
(7)
Where Ksp is solubility constant of Calcium carbonate, K is the acid dissociation constant of HCO3− and S is the solubility of Calcium carbonate. Using 4-7 equations we can derive an equation for the solubility of calcium carbonate which is as follows in the equation 8.
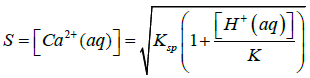 (8)
(8)
Here K and Ksp values of vaterite polymorph of Calcium Carbonate should be taken at 37°C which is the normal body temperature. In this equation 8 it undoubtedly shows that the solubility of Calcium Carbonate is increasing when the [H+(aq)] of the solution is increased or in other words if the pH of the solution is decreased [29].
Commercially available Cisplatin Injection which was used for the research is a clear, colourless to pale yellow colour, sterile aqueous solution. Each 50 mL cisplatin Injection comprises 1 mg/mL Cisplatin, 9 mg/mL sodium chloride, mannitol, sodium hydroxide and/or hydrochloric acid to regulate pH, and water for injection to a final volume of 50 ml. The pH range of Cisplatin Injection is about 3.5 to 5.0 [30]. So normally anticancer drug Cisplatin is stable in acidic pH values. Generally cancerous cells are low in pH value. There are several different explanations but normally due to higher rate of cell differentiation, there is erratic and uncontrolled growth where the oxygen supply is lower. Hence the tumour cells undergo anaerobic respiration resulting into formation of lactic acid. This event changes the pH profile of tumour cells. So the instability nature of host material porous CaCO3 nanoparticles at low pH values can be used as a mechanism of targeted drug delivery and releasing at the acidic cancerous cells.
It is vividly shown in Figure 9a where anticancer drug release profiles for each different pH and time intervals prefer low pH values in releasing the drug from CaCO3 nanoparticles. During the first 5 h all 3 acidic pH values show 5% of release and after 10 h it becomes approximately 10% of release of cisplatin. At the end of 24 h 12%, 14% and 17% of anticancer drug was released from the porous CaCO3 nanoparticle which is in pH=6, pH=5, and pH=4 respectively. This signifies that, still the anticancer drug cisplatin is retained within porous calcium carbonate nanoparticle and even under fairly strong acidic conditions slow release can be accomplished. Furthermore when considering the pH of the vicinity for cancer cell (pH ~5-6), for the pH 6.0 buffer solution, about 5% of release is observed during the first 5 h. Over the span of 10 h, the release has only reached a percentage of approximately 9%. At the end of 24 h period, it is evident that it has reached of nearly 12% retaining the drug in the porous material for further slow release over time. Nevertheless, at pH 5.0, 5% of release is reached during the first 5 h of study. Roughly, 10% of release was elicited during the first 10 h and ascended to pitch a value just above 13%, at the end of 24 h study period. A negligible amount of release of anticancer drug is observed at pH 7.0 which corresponds to the pH of healthy tissues in our body (pH=6.8-7.3). In contrast, elicit release can be observed at all acidic pH values used (4.0, 5.0 and 6.0). So the anticancer drug cisplatin is targeted.
The main threats experienced during cisplatin therapy and over dosage comprise nephrotoxicity, myelosuppression, electrolyte disturbances, neurotoxicity, anaphylactic reactions, and ototoxicity. Nausea and vomiting can be common symptoms. Most effects of over dosage are not typically seen instantaneously, but occur numerous days to months after the event [31]. The reasons of death from an overdose from cisplatin contain myelosuppression, renal failure and tetany. So if the dose we use is not delivered in a controlled manner these kinds of treats can be experienced by the patient. In that case controlled drug release coupled with constant minimum amount of slow releasing drug is important in drug delivery system architecture. On the other hand cisplatin decay mono exponentially with a half-life of about 20 to 30 min following bolus administrations of 50 or 100 mg/m2 doses [32]. To achieve sustained release formulation, drug must enter into the blood circulation system at nearly the similar rate at which it is eliminated. Each and every drug has its own characteristic properties related to elimination rate, which is the addition of all elimination processes, normally include metabolism, urinary excretion and all the process that enduringly remove drug from the blood stream. Drugs like cisplatin with shorter half-life less than 2 h are poor candidate for sustained release formulation. On the other hand drugs which possess longer half-life more than 8 h are also poor candidate in SR formulation, since the effect is already sustained [33]. So it is very much important to maintain therapeutic blood levels over an extended period of time. Drug encapsulated porous CaCO3 Nano carrier will help the drug to increase its half-life to preferable range. When considering Figure 9b it is very much obvious that within the time intervals the amount of drug released is mostly uniform and approximately a constant. So very minimum amount of drug (ppb range) is released from the host carrier porous CaCO3 nanoparticles at a constant rate within time intervals. Therefore it is advantageous in targeted drug delivery to cancerous cells where minimum, approximately constant amount slow release can be achieved.
XRF, XRD, FTIR studies revealed that the spherical thermodynamically unstable vaterite porous CaCO3 nanoparticles were synthesized using precipitation reaction of NaHCO3 with Ca(CH3COO)2 in H2O/EG media. FTIR spectra show interactions between Porous Calcium Carbonate nanoparticles and the anticancer drug cisplatin. SEM images show that there is hardly any morphological difference between Porous Calcium Carbonate nanoparticles and the anticancer drug encapsulated product indicating that the drug is not resident on the surface but has been encapsulated within the pores of the nanoparticles. SEM images also give evidence to the preserving of the spherical morphological structure of vaterite polymorph in the encapsulated product.
EDAX spectrum gives a clear evidence to prove that cisplatin is encapsulated in the centres of nanoparticles. The colloidal particles are in nano-range with very narrow particle size distribution. Cisplatin was successfully encapsulated in Porous Calcium Carbonate nanoparticles which retain its chemical identity within the confined environment explained by XRF studies. FTIR data give exactly the same literature peak values for Vaterite Calcium Carbonate polymorph and it shows that cisplatin is encapsulated inside the nanoparticles.
By analysing data from in vitro drug release studies hardly any release of cisplatin at neutral and basic pH values and rate of release increases with decreasing pH or in acidic pH values. At physiological pH of blood and that of healthy cells cisplatin is not released while at mildly acidic pH values of cancer cells cisplatin is slowly released. The encapsulated product can be delivered to cancer cells in a targeted safe manner and the highly toxic anticancer drug can be released in minimum approximately a constant sufficient dosage only at the vicinity of the cancer cells without harming the healthy cells.