Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Research Article - (2017) Volume 6, Issue 1
In present study, some 4-piperidinopiperidine (PP) and 4-amino methylpiperidine (AMP) derivatives (PP1-3 and AMP4-9) have been synthesized to explore their analgesic potential. Activity of compounds evaluated by in-vivo thermal (tail immersion) method produced significant analgesia at different doses. Docking results explained good binding affinity of synthesized derivatives and potential interaction of all compounds with mu-opioid receptor. The pharmacophoric model of synthesized compounds showed possible structural features required for analgesic activity when compared with standards (Fentanyl, Morphine, Pethidine). Among all PP1, AMP5 and AMP6 emerged out as potent analgesic agents.
Graphical abstract

Keywords: 4-Piperidino piperidine, 4-(Amino methyl) piperidine, Tail-immersion assay, Possible maximal analgesic percentage, Morphine opioid receptor
Effective pain management has always been the deliberating task for scientists. Two major classes of traditional analgesics including nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids are mainly used in treatment of pain [1,2]. Studies revealed that the analgesic potential of narcotic drugs is closely and strongly associated with binding of G-protein coupled opioid receptors (OR) in central nervous system (CNS), especially mu-opioid receptors (MOR). Reported adverse effects mainly; addiction, tolerance, dependence and abuse potential limited the clinical use of opioid drugs [3,4]. Therefore, scientists focused on finding novel compounds having effective analgesic potential with limited side effects [5-8].
In this regard many derivatives of morphine were developed to enhance therapeutic potential and lessen side effects by slight modification. Thus, exhibiting better activity than morphine [9-11]. Structural activity relationship (SAR) of morphine revealed that the presence of piperidine ring is necessary for analgesic activity [12-14].
Piperidine possesses enormous biological and pharmacological potential and presence of piperidine ring in various clinically used drugs reflects its importance in drug design. Pethidine, fantanyl, ohmefentanyl, remifentanyl, ketobemidon and a variety of molecules contain piperidine nucleus and used as effective analgesics.
Extensive research presented that the substituted piperidine molecule showed potential therapeutic properties, good receptor binding and revealed as a leading nucleus with potent pharmacological actions therefore, widely used for the management of pain and inflammation. Recently, series of piperidine derivatives have been reported showing significant antinociceptive activity [15-18]. Various novel substituted piperidines have been prepared in our lab and most of the derivatives displayed potent analgesic activity [18-27].
Advancement in computational technique helps in predicting binding modes and affinity of compounds with the receptor. Many scientists have already investigated the binding properties of different ligands at the μ, κ and δ−opioid receptors [28-30]. We have used the available crystal of Mus musculus μ opioid receptor (MOR) protein (PDB: 4DKL). That has importance in preclinical research on pain management, but has been associated with the addictive side effects of opiates and even alcoholism [31]. Hence, the main goal of the current study is to synthesize effective analgesic agents having piperidine nucleus and identify significant structural features of the synthesized derivatives for possible interaction with μ opioid receptor. A focus on the binding properties of derivatives may provide insights of interest for drug design and discovery procedures.
Analgesic activity
Analgesic response is presented in Table 1 and possible maximal analgesic percentage (PMAP) in Table 2. All derivatives PP1-3 and AMP4-9 exhibited better analgesia than parents PP and AMP. Derivatives of PP showed analgesic potential more than standard (Pethidine) at 50 mg/kg while derivatives of AMP demonstrated more potent analgesia at low tested doses (1 mg/kg and 0.1 mg/kg).
| Compounds | Dose mg/kg |
Analgesia TFLD (mean increase in latency after drug administration ± SEM) | |||||
|---|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | ||
| Control (a) | 2.50 ± 0.06 | 2.49 ± 0.22 | 2.42 ± 0.20 | 1.92 ± 0.11 | 2.15 ± 0.17 | 2.59 ± 0.24 | |
| Control (b) | 50 (p.o.) |
0.99 ± 0.05 | 0.88 ± 0.03 | 0.90 ± 0.01 | 0.86 ± 0.24 | 0.94 ± 0.04 | 0.95 ± 0.04 |
| Control (c) | 2.05 ± 0.09 | 1.64 ± 0.20 | 2.20 ± 0.30 | 2.29 ± 0.17 | 2.42 ± 0.22 | 2.45 ± 0.22 | |
| PP (a) | 2.46 ± 0.15 | 3.59** ± 0.02 | 3.38* ± 0.05 | 2.58* ± 0.13 | 2.30 ± 0.04 | 1.62 ± 0.28 | |
| PP1 (b) | 6.09** ± 0.31 | 7.99** ± 0.34 | 8.89** ± 0.23 | 4.39** ± 0.12 | 3.39** ± 0.09 | 2.86** ± 0.12 | |
| PP2 (c) | 5.63** ± 0.21 | 3.80** ± 0.20 | 3.00** ± 0.16 | 2.83* ± 0.34 | 2.37 ± 0.18 | 2.33 ± 0.27 | |
| PP3 (c) | 3.42** ± 0.09 | 3.21** ± 0.05 | 2.95** ± 0.18 | 2.47* ± 0.14 | 2.27 ± 0.13 | 2.20 ± 0.20 | |
| Control (d) | 50 (i.p.) |
1.30 ± 0.031 | 1.37 ± 0.26 | 1.37 ± 0.30 | 1.01 ± 0.13 | 1.41 ± 0.07 | 1.79 ± 0.13 |
| PethidineHCl (d) | 2.84** ± 0.14 | 3.81** ± 0.11 | 2.95** ± 0.09 | 2.12** ± 0.08 | 1.38 ± 0.08 | 1.56 ± 0.09 | |
| Control (e) | 1 (i.p) |
1.15 ± 0.12 | 1.22 ± 0.08 | 1.32 ± 0.06 | 1.32 ± 0.03 | 1.19 ± 0.12 | 1.33 ± 0.09 |
| Control (f) | 0.98 ± 0.003 | 0.99 ± 0.007 | 1.22 ± 0.031 | 1.22 ± 0.06 | 1.18 ± 0.01 | 1.32 ± 0.01 | |
| AMP (e) | 1.10 ± 0.041 | 1.19 ± 0.06 | 1.25 ± 0.025 | 1.26 ± 0.03 | 1.10 ± 0.12 | 1.26 ± 0.05 | |
| AMP5 (e) | 2.82** ± 0.15 | 2.99** ± 0.18 | 2.98** ± 0.29 | 1.71** ± 0.15 | 1.58 ± 0.11 | 1.48 ± 0.15 | |
| AMP6 (e) | 3.17** ± 0.29 | 2.79** ± 0.08 | 1.66** ± 0.12 | 1.47** ± 0.04 | 1.37 ± 0.04 | 1.39 ± 0.06 | |
| AMP7 (f) | 3.48** ± 0.10 | 2.48** ± 0.08 | 1.30* ± 0.05 | 1.28 ± 0.09 | 1.58 ± 0.11 | 1.64 ± 0.09 | |
| AMP8 (f) | 0.94 ± 0.06 | 2.99** ± 0.27 | 2.78** ± 0.05 | 1.22 ± 0.04 | 1.17 ± 0.05 | 1.55 ± 0.12 | |
| AMP9(f) | 2.49** ± 0.21 | 2.81** ± 0.05 | 1.41* ± 0.16 | 1.19 ± 0.14 | 1.26 ± 0.08 | 1.57 ± 0.18 | |
| AMP4 (f) | 0.1 (i.p) |
2.61** ± 0.21 | 2.57** ± 0.16 | 2.67** ± 0.11 | 1.20 ± 0.01 | 1.37 ± 0.04 | 1.56 ± 0.09 |
Table 1: Analgesic effect of 4'-Piperidinopiperidine (PP), 4-(amino methyl) piperidine (AMP) and their derivatives (PP 1-3 and AMP 4-9) by tail immersion method. Note: n/group= 10, Significant difference by student’s t test: *p<0.05, **p<0.01 as compared to control PP.
| Compounds | Dose mg/kg |
Possible maximal analgesic percentage (PMAP) | |||||
|---|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | ||
| PP | 50 (p.o.) | 1.47 | 3.41 | 3.05 | 1.68 | 1.2 | 0.03 |
| PP1 | 8.7 | 11.92 | 13.45 | 5.83 | 4.13 | 3.23 | |
| PP2 | 5.93 | 2.77 | 1.38 | 1.09 | 0.29 | 0.22 | |
| PP3 | 2.45 | 2.09 | 1.64 | 0.81 | 0.47 | 0.34 | |
| PethidineHCl | 3.14 | 4.77 | 3.31 | 1.91 | 0.66 | 0.62 | |
| AMP | 1(i.p.) | 0.24 | 0.39 | 0.49 | 0.51 | 0.24 | 0.12 |
| AMP5 | 3.83 | 3.57 | 3.46 | 1.78 | 0.99 | 0.82 | |
| AMP6 | 3.08 | 3.38 | 3.39 | 1.21 | 0.78 | 0.81 | |
| AMP7 | 2.23 | 2.14 | 1.55 | 1.51 | 1.02 | 1.12 | |
| AMP8 | 1.25 | 2.22 | 2.16 | 1.21 | 0.77 | 0.12 | |
| AMP9 | 2.48 | 2.34 | 1.65 | 1.27 | 0.40 | 0.22 | |
| AMP4 | 0.1(i.p.) | 2.64 | 2.67 | 2.74 | 1.25 | 0.85 | 0.52 |
Table 2: Possible Maximal Analgesic Percentage (PMAP) of 4'-Piperidinopiperidine (PP), 4-(amino methyl) piperidine (AMP) and their derivatives (PP1-3 and AMP4-9) by tail immersion method.
PP1, AMP5 and AMP6 exhibited highly significant analgesia with maximum duration of action. PP1 emerged out as prominent analgesic agent than parent and standards with persistent action till 180 minutes at 50 mg/kg dose. Dinitro benzoyl derivative AMP7 exhibited potent analgesia with rapid onset of highly significant effect which persisted till 60 minute than became significant at 90 minute. AMP8 showed a gradual increase in analgesia from 60 to 90 minute then its analgesic effect became insignificant. Bromobenzyl derivative AMP9 displayed nociception similar to AMP7. Bromo phenacyl derivative PP2 and alkyl derivative PP3 displayed highly significant response till 90 minute.
Substituted phenacyl (PP1, AMP5, AMP6) and benzoyl derivative (AMP7) exhibited pronounced analgesia with quick onset and long duration of action at low tested doses. The meta- dinitro benzoyl derivative AMP7 and para- bromo benzyl AMP9 exhibited highly significant analgesic activity as compared to para- bromo benzoyl derivative AMP8.
PMAP exhibited by derivatives of PP (0.22-13.45%) is in order of PP1>PP2>PP3 at the dose of 50 mg/kg while percentage analgesia displayed by AMP4-9 (0.12-3.83%) as represented in Table 2 is in order of AMP5>AMP6>AMP4>AMP9>AMP7>AMP8>at the various tested doses.
Pharmacophoric study
All synthesized derivatives showed equal or more pharmacophoric regions than standards and parents as given in Table 3.
| Compounds | AR | H | HBA | HBD | PI | Total |
|---|---|---|---|---|---|---|
| Fentanyl | 2 | 2 | 1 | 0 | 1 | 6 |
| Morphine | 1 | 2 | 3 | 2 | 1 | 9 |
| Pethidine | 1 | 2 | 1 | 0 | 1 | 5 |
| PP | 0 | 0 | 0 | 1 | 2 | 3 |
| PP1 | 1 | 0 | 3 | 1 | 2 | 7 |
| PP2 | 1 | 2 | 1 | 1 | 2 | 7 |
| PP3 | 0 | 1 | 0 | 1 | 2 | 4 |
| AMP | 0 | 0 | 0 | 2 | 2 | 4 |
| AMP4 | 2 | 4 | 2 | 2 | 2 | 12 |
| AMP5 | 2 | 0 | 6 | 2 | 2 | 12 |
| AMP6 | 2 | 2 | 2 | 2 | 2 | 10 |
| AMP7 | 2 | 0 | 10 | 1 | 0 | 13 |
| AMP8 | 2 | 4 | 2 | 1 | 0 | 9 |
| AMP9 | 2 | 4 | 0 | 2 | 2 | 10 |
Table 3: Pharmacophoric features of standard drugs (Fentanyl, Morphine, Pethidine), PP, AMP and their analogues (PP1-3 and AMP4-9) using Ligand Scout 3.02. Note: AR=Aromatic region, H=Hydrophobic region, HBA=Hydrogen bond acceptor, HBD=Hydrogen bond donor, PI=Positive ionizable group.
Five pharmacophoric features (aromatic region, hydrophobic regions, hydrogen bond acceptor, positive ionic interaction) are shared by Fentanyl, Pethidine and Morphine. All derivatives shared maximum pharmacophoric features with Morphine as clear from Table 4 and Figure 1.
Figure 1: Shared Pharmacophoric features, blue-ring=aromatic ring, blue star=positive ion, red circle=hydrogen bond acceptor, green circle=hydrogen bond donor, yellow circle=hydrophobic regions. (a): Fentanyl, Pethidine with Morphine. (b): PP1, PP2 with Morphine. (c): AMP4, AMP6 with Morphine. (d): AMP5, AMP7 with Morphine, calculated by chimera 1.10.
| Compounds | AR | H | HBA | HBD | PI | Total |
|---|---|---|---|---|---|---|
| Fentanyl, Pethidine with Morphine | 1 | 2 | 1 | 0 | 1 | 5 |
| Fentanyl PP1, PP2 | 1 | 0 | 1 | 0 | 1 | 3 |
| Fentanyl PP, PP3 | 0 | 0 | 0 | 0 | 1 | 1 |
| Morphine, PP1, PP2 | 1 | 0 | 1 | 1 | 1 | 4 |
| Morphine, PP, PP3 | 0 | 0 | 0 | 1 | 1 | 2 |
| Pethidine PP1, PP2 | 1 | 0 | 1 | 0 | 1 | 3 |
| Pethidine PP, PP3 | 0 | 0 | 0 | 0 | 1 | 1 |
| Fentanyl AMP4, AMP6 | 2 | 2 | 1 | 0 | 1 | 6 |
| Fentanyl AMP5, AMP7 | 2 | 0 | 1 | 0 | 0 | 3 |
| Fentanyl AMP8, AMP9 | 2 | 2 | 0 | 0 | 0 | 4 |
| Morphine AMP4, AMP6 | 1 | 2 | 2 | 2 | 1 | 8 |
| Morphine AMP5, AMP7 | 1 | 0 | 3 | 1 | 0 | 5 |
| Morphine AMP8, AMP9 | 1 | 2 | 0 | 1 | 0 | 4 |
| Pethidine AMP4, AMP6 | 1 | 2 | 1 | 0 | 1 | 5 |
| Pethidine AMP5, AMP7 | 1 | 0 | 1 | 0 | 0 | 2 |
| Pethidine AMP8, AMP9 | 1 | 2 | 0 | 0 | 0 | 3 |
Table 4: Shared pharmacophoric features of standard drugs (Morphine, Pethidine, Fentanyl), PP, AMP and their analogues (PP1-3 and AMP4-9) using Ligand Scout 3.02 Note: AR=Aromatic region, H=Hydrophobic region, HBA=Hydrogen bond acceptor, HBD=Hydrogen bond donor, PI=Positive ionizable group.
PP1, PP2 shared 4 pharmacophoric features (aromatic region, hydrogen bond acceptor, hydrogen bond donor, positive ionic interaction) and eight features (aromatic region, hydrophobic regions, hydrogen bond acceptors, hydrogen bond donors, positive ionic interaction) are shared by AMP4, AMP6 with morphine. Table 5 showing that all the derivatives following ‘Lipinski’s rule of 5’ in term of Log P, molecular weight, hydrogen bond donor and acceptor criteria showing the possibility for molecules to be good drug candidates [32].
| Ligand | Binding Affinity (Kcal/Mol) |
Interacting Amino Acids Residues | Lipinski’s Rule Of Five | ||||
|---|---|---|---|---|---|---|---|
| Hydrophobic Interactions | Hydrogen Bonding | Mol. Weight | Logp | H-B Donor | H-B Acceptor | ||
| Fentanyl | -8.3 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | - | 336 | 3.89 ± 0.53 | 0 | 3 |
| Morphine | -7.6 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | Met151, His297 | 285 | 0.43 ± 0.66 | 2 | 4 |
| Pethidine | -6.2 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | His297, Tyr148 | 247 | 2.35 ± 0.37 | 0 | 3 |
| PP | -6.1 | Gly325, Ile296, Me t151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | - | 168 | 1.53 ± 0.3 | 1 | 2 |
| PP1 | -6.6 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | Tyr326 | 331 | 3.15 ± 0.43 | 0 | 6 |
| PP2 | -7 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322 | Tyr326 | 365 | 4.16 ± 0.5 | 0 | 3 |
| PP3 | -6.3 | Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr1, Tyr326, Ile322, Lys233, Lys303 | - | 224 | 3.33 ± 0.32 | 0 | 2 |
| AMP | -4.3 | Gly325, Ile296, Met151, Trp293, His297, Trp318, Tyr148, Tyr326, Ile322, Lys233 | Asp147, Ile322 | 114 | -0.19 ± 0.25 | 3 | 2 |
| AMP4 | -7.3 | Gly325, Ile296, Met151, Trp293, His297, Val236, Trp318, Tyr148, Tyr326, Ile322, Lys233 | Tyr148 | 508 | 4.92 ± 0.6 | 1 | 4 |
| AMP5 | -8.4 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | Tyr148, Tyr326 | 440 | 2.9 ± 0.49 | 1 | 10 |
| AMP6 | -8.2 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | - | 378 | 4.09 ± 0.47 | 1 | 4 |
| AMP7 | -8.5 | Gly325, Ile296, Met151, Trp293, His297, Val236, Trp318, Tyr148, Tyr326, Ile322, Lys233 | - | 502 | 1.36 ± 0.47 | 1 | 16 |
| AMP8 | -7.4 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | - | 480 | 3.72 ± 0.56 | 1 | 4 |
| AMP9 | -6.5 | Gly325, Ile296, Met151, Trp293, His297, Val236, Val300, Trp318, Tyr148, Tyr326, Ile322, Lys233 | - | 452 | 5.36 ± 0.53 | 1 | 2 |
Table 5: Docking score, interactions and Lipinski’s rule of five of standards and synthesized compounds using PDB ID: 4DKL, using Chimera 1.10.2 Note: *Hydrogen bonding and label colored by yellow dotted lines and residue involved colored by black *Hydrophobic residues labeled by blue color.
Docking study
Binding of standards and derivatives with μ-opioid receptor (MOR) studied and represented in Table 5 showing binding energy as well as amino acid residues involved in hydrogen and hydrophobic interactions.
Previously reported in-silico studies providing significant information and insight about amino acid residues and type of interactions involved in ligand-target binding with the number of synthesized molecules and standard drugs including morphine, fentanyl, etorphine, oxymorphone, naloxone and naltrexone [33,34]. Present study is in agreement with the earlier findings indicating the involvement of same amino acid residues for good binding. Asp147, Tyr148 and Tyr326 formed polar and hydrophobic interactions with parents, standards and derivatives. Tyr148 and Tyr326 were involved in Hydrogen bonding with Pethidine, PP1, PP2, AMP4 and AMP5 as presented in Figure 2.
All the synthesized derivatives showed potential binding with μ- opioid receptor, specially, nitro phenacyl (PP1, AMP5) and propiophenone (AMP6) derivatives. They showed effective binding with amino acid residues through hydrogen bonding and hydrophobic interactions justifying significant response with early onset and long duration of action at lower dose and can be taken for further testing and modification to get better analgesic agents. Overall, pharmacophoric study of synthesized compounds and standards showing the presence of shared features providing possible justification of the good results of compounds for analgesic activity.
Chemicals, reagents and instruments
Chemicals were purchased from Sigma and solvents used were of E. Merck. TLC (E. Merck) with pre-coated silica gel were used for monitoring reactions and were visualized under UV light at 254 nm and 366 nm on HP-UVIS Desaga (Heidelberg). Iodine vapors were also employed for the detection of spots on TLC plate. Melting points were taken in capillary tubes (haematocrit capillary) on STUART melting point instrument and were uncorrected, anhydrous silica from E. Merck was used for drying reaction product.
Nuclear magnetic resonance H1-NMR spectra were recorded in d6- DMSO on Bruker Advance AV-300 (France) spectrometer operating at 300-500 Megahertz (MHz) using tetra methyl silane (TMS) as internal standard. Mass spectra (MS) were obtained on JEOL JMS-H x 110 spectrometer. Infra-Red (IR) spectra were measured on FTIR-8900 Shimadzu spectrophotometer using KBr disc. Ultraviolet (UV) spectra were recorded in methanol/DMSO on a CECIL (CE 7200)/Hitachi U-3200 spectrophotometer.
General method for synthesis of compound (PP1-3 and AMP4-9)
Solutions of 4'-Piperidinopiperidine (PP) (0.005M) and 4-(amino methyl) piperidine AMP (0.005M) were mixed with substituted phenacyl, benzyl, benzoyl halides and 2-bromobutane (0.005-0.01M) in acetone with few drops of triethylamine. Solution stirred at room temperature and then refluxed at 50-52°C for 10-50 hours (Schemes 1-3). Reactions were monitored with TLC. Products obtained were solid or gummy solid in nature, washed with acetone and/or purified by recrystallization by using single or double solvent system. The pure compound was dried in vacuum desicator over anhydrous silica.
2-(4'-nitrophenyl)-2-oxoethyl-4-(piperidinyl)piperidinium bromide (PP1): Brownish yellow powder, Yield: 77.07%, Solubility: DMSO, IR (KBr) νmax (cm−1): 3440.8, 2937.4, 1676.0, 1600.8, 1521.7, 1458.1, 1431.1, 1350.1, 1247.9, UV λmax (MeOH) nm: 263.2, 1H-NMR (d6- DMSO, 300 MHz) δ: 8.372 (d, 2H, J=8.7 MHz, H-3', H-5'), 8.292 (d, 2H, J=9.0 MHz, H-2', H-6'), 3.324 (s, 2H, H-1'''), 2.575-2.490 (m, 1H, H-4), 2.429-1.979 (m, 8H, H-2, H-6, H-2'', H-6''), 1.756-1.018 (m, 10H, H-3'', H-4'', H-5'', H-3, H-5), EIMS m/z: 311 (M+ - HBr, C18H25N3O3) 245, 167.
2-(4'-bromophenyl)-2-oxoethyl-4-(piperidinyl)piperidinium bromide (PP2):Brown powder, Yield: 71.78%, Solubility: DMSO, CHCl3, IR (KBr) νmax (cm−1): 3404.1, 2931.6, 1679.9, 1587.3, 1483.2, 1425.3, 1394.4, 1232.4, 678.9, UV λmax (MeOH) nm: 241.2, 1H-NMR (d6-DMSO, 300 MHz) δ: 7.857 (d, 2H, J=8.1 MHz, H-3', H-5'), 7.695 (d, H, J=8.1 MHz, H-2', H-6'), 3.333 (s, 2H, H-1'''), 2.715 (m, 1H, H-4), 2.259-1.828 (m, 8H, H-2, H-6, H-2'', H-6''), 1.494–1.019 (m, 10H, H-3'', H-4'', H-5'', H-3, H-5), EIMS m/z: 365 (M+ - HBr, C18H25N2OBr) 282, 241, 182, 170.
1-(2-butyl)-4-(piperidinyl)piperidinium bromide (PP3): Brownish oily compound, Yeild: 93.81%, Solubility: CH3OH, C2H5OH, Ether, H2O, DMSO, IR (KBr) νmax (cm−1): 3381.68, 1446.57, 1403.73, 1274.68, UV λmax (MeOH) nm: 221.2, 1H-NMR (d6-DMSO, 500 MHz) δ: 2.988-2.932 (m, 1H, H-3'), 2.775-2.726 (m, 1H, H-4), 2.530-2.432 (m, 8H, H-2'', H-6'', H-2, H-6), 1.465-1.155 (m, 12H, H-3'', H-4'', H-5'', H-3, H-5, H-2'), 1.003 (d, 3H, J=5.5 MHz, H-4'), 0.955-0.801 (m, 3H, H-1'), EIMS m/z: 224 (M+ - HBr, C14H28N2) 210, 196.
1-[2-(4-bromophenyl)-2-oxoethyl]-4-({[2-(4-bromophenyl)-2- oxoethyl]azaniumyl}methyl)piperidin- 1-ium-di-bromide (AMP4): Brown gummy solid, Yield: 52.08%, Solubility: CH3OH, C2H5OH, DMSO, IR (KBr) νmax (cm−1): 3358.2, 1701.3, 1645.4, 1454.4, 1369.1, 811.8, 612.7, UV λmax (MeOH) nm: 206, 238, 1HNMR (d6-DMSO, 400 MHz) δ: 1.009-0.947 (q, 4H, H-3, H-5), 1.342-1.307 (m, 1H, H-4), 2.495-2.486 (t, 4H, H-2, H-6, J=3.6 Hz), 2.719-2.703 (d, 2H, H-7, J=6.4 Hz), 2.837 (s, 2H, H-7'), 3.299 (s, 2H, H-7''), 7.629-7.602 (d, 4H, H-3', H-5', H-3'', H-5'', J=8.4 Hz), 7.833-7.813 (d, 4H, H-2', H-6', H-2'', H-6'', J=8 Hz), FAB m/z: 511.07 (M+ - 2HBr), 354, 275, 197.
2-(4-nitrophenyl)-2-oxoethyl]-4-({[2-(4-nitrophenyl)-2- oxoethyl]azaniumyl}methyl)-piperidin-1-ium dibromide (AMP5): Brown gummy solid, Yield: 45.8%, Solubility: C2H5OH, DMSO, IR (KBr) νmax (cm−1): 3413.8, 1627.8, 1585.4, 1448.4, 1377.1, 1342.4, 819.7, UV λmax (MeOH) nm: 225, 265, 1HNMR (d6-DMSO, 400 MHz) δ:1.387-1.291 (m, 1H, H-4), 1.896-1.840 (q, 4H, H-3, H-5), 2.495-2.486 (t, 4H, H-2, H-6, J=3.6 Hz), 2.748-2.731 (d, 2H, H-7, J=6.8 Hz), 2.874 (s, 2H, H-7'), 3.294 (s, 2H, H-7''), 8.082-8.061 (d, 4H, H-2', H-6', H-2'', H-6'' J=8.4 Hz), 8.208-8.187 (d, 4H, H-3', H-5', H-3'', H-5'', J=8.4 Hz), FAB m/z: 440.27 (M+ - 2HBr), 348, 277, 259, 245.
1-(1-oxo-1-phenylpropan-2-yl)-4-{[(1-oxo-1-phenylpropan-2- yl)azaniumyl]methyl}piperidin-1-ium dibromide (AMP 6): Brown gummy solid, Yield: 32.24%, Solubility: CH3OH, C2H5OH, H2O, DMSO, IR (KBr) νmax (cm−1): 3429.2, 1685.7, 1546.8, 1444.6, 1380.9, 765.7, UV λmax (MeOH) nm: 205, 250, 1HNMR (d6-DMSO, 400 MHz) δ: 1.296 (s, 6H, H-8', H-8''), 1.362-1.327(d, 4H, H-3, H-5, J=14 Hz), 1.868-1.835 (d, 1H, H-4, J=13.2 Hz), 2.494-2.486 (t, 4H, H-2, H-6, J=3.2 Hz), 2.743 (s, 2H, H-7), 2.869 (s,1H, H-7''), 3.294 (s, 1H, H-7'), 7.510-7.471 (t, 4H, H-3', H-5', H-3'', H-5'', J=15.6 Hz), 7.632-7.595 (t, 2H, H-4', H-4'', J=14.8 Hz), 7.944-7.926 (d, 4H, H-2', H-6', H-2'', H-6'', J=7.2 Hz), FAB m/z: 378 (M+ - 2HBr), 363, 348, 301, 272, 231, 194.
N-{[1-(3,5-dinitrobenzoyl)piperidin-4-yl]methyl}-3,5- dinitrobenzamide (AMP 7): Light Brown powder, Yield: 34.62%, Solubility: C2H5OH, DMSO, IR (KBr) νmax (cm−1): 3095.5, 1672.2, 1624, 1537.2, 1481.2, 1440.7, 1346.2, UV λmax (MeOH) nm: 207, 229, 1HNMR (d6-DMSO, 400 MHz) δ: 1.387-1.319 (q, 4H, H-3, H-5), 2.746 - 2.716 (t, 2H, H-7, J=12 Hz), 2.865-2.797 (m, 2H, H-4), 4.481-4.454 (d, 4H, H-2, H-6, J=10.8 Hz), 9.057-9.052 (d, 4H, H-2', H-6', H-2'', H-6'', J=2 Hz), 9.278- 9.250 (t, 2H, H-4', H-4'', J=11.2 Hz), FAB m/z: 503 (M+ - 2HCl), 475, 411, 333, 305, 276, 241.
4-bromo-N-{[1-(4-bromobenzoyl)piperidin-4-yl]methyl}benzamide (AMP 8): Brown powder, Yield: 27.80%, Solubility: CH3OH, C2H5OH, DMSO, IR (KBr) νmax (cm−1): 1591.2, 1444.6, 1311.5, 1114.8, 829.3, 653.8, UV λmax (MeOH) nm: 205, 233, 1HNMR (d6-DMSO, 400 MHz) δ: 1.896- 1.847 (q, 4H, H-3, H-5), 2.833- 2.770 (m, 1H, H-4), 3.100-3.164-3.122 (d, 2H, H-7, J=16.8 Hz), 3.298-3.217 (t, 4H, H-2, H-6, J=13.2 Hz), 7.793-7.772 (d, 4H, H-3', H-5', H-3'', H-5'', J=8.4 Hz), 7.330-7.309 (d, 4H, H-2', H-6', H-2'', H-6'', J=8.4 Hz), FAB m/z: 480 (M + - 2HCl), 400, 323, 297.
1-[(4-bromophenyl)methyl]-4-({[(4- bromophenyl)methyl]azaniumyl}methyl) piperidin-1-ium dibromide (AMP9): Brown gummy solid, Yield: 12.56%, IR (KBr) νmax (cm−1): 3413.8, 1539.1, 1452.3, 1346.2, 806.2, 597.9, UV λmax (MeOH) nm: 203, 225, 1HNMR (d6-DMSO, 400 MHz) δ: 1.372-1.28 (q, 4H, H-3, H-5), 1.879-1.791 (m, 1H, H-4), 2.494-2.486 (t, 4H, H-2, H-6, J=3.2 Hz), 2.923-2.883 (d, 2H, H-7, J=16 Hz), 3.62 (s, 2H, H-7'), 4.825 (s, 2H, H-7''), 7.572-7.551 (d, 4H, H-2', H-6', H-2'', H-6'', J=8.4 Hz), 7.767-7.736 (d, 4H, H-3', H-5', H-3'', H-5'', J=12.4 Hz), FAB m/z: 454 (M+ - 2HBr), 374, 284, 268, 113.
Analgesic activity
Antinociception was evaluated by measuring tail flick latency time by tail-immersion assay (thermal method) [35,36]. Mice of either sex between 20-25 gm were divided into groups of ten animals. Test compounds were administered orally (p.o.) as 1% tragacanth suspension and intraperitoneally (i.p.) in DMSO (40%) at different doses, i.e., 50 mg/kg, 1 mg/kg and 0.1 mg/kg. Analgesic activity was expressed as TFLD ± S.E.M. in seconds. Significant differences between means were determined by a student’s t-test and values of p<0.05 were considered as significant while p<0.01 was highly significant. TFLD was calculated as:
Analgesia TFLD=(Post-drug TFL – Pre-drug TFL)
The results were also expressed as possible maximal analgesic percentage (PMAP) [37].
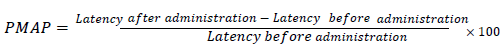
Pharmacophoric studies
The structure-based pharmacophore models were generated and compounds were compared with standards (Fentanyl, Morphine, Pethidine) for same structural features using the Ligand Scout 3.12 software package [38] which interpreted possible sites for ligandreceptor interactions such as charge transfer, aromatic region/s (AR), hydrogen bond donor/s (HBD), hydrogen bond acceptor/s (HBA), hydrophobic (H) and positive ionic interaction (PI).
Docking studies
The mol files of standards (Fentanyl, Morphine, Pethidine) were obtained from CHEM SPIDER database. Structures of compounds were drawn using Marvin Sketch 5.8 and saved in mol format. Energy minimization was done by Open Babel; force field applied mmff 94 with optimization algorithm steepest descent upto 500 steps and saved in PDB format. The X-ray crystal structure for MOR (4DKL) was taken from PDB Data Bank. All ligands, heteroatoms and other crystallographic agents were removed from the original proteins structure, energy minimization done upto 1000 steepest descent steps, hydrogens were added, Gasteiger-Hückel charges were assigned using AMBERff 14SB.
Docking calculations were done by Auto Dock Vina 0.9.2 [39] with default parameters except exhaustiveness value which was set to 10. The grid box of dimension 11.987 × 18.153 × 14.004 Å (x, y, and z) and Center -27.794 × -12.782 × -11.771 Å (x, y, and z) was assigned on the receptor binding pocket. In order to evaluate the docking procedure, the respective crystallographic ligand of the target was re-docked over the active site that testified the accuracy of the docking calculations. Docked poses with lowest binding affinity (kcal/mol) and RMSD value between (0.5-3) were saved in pdf format.
Chimera 1.10.2 used for observing possible interactions between the ligands and the active site residues of the receptor.
Research grant of University of Karachi, Karachi University, Pakistan.
All the authors have no conflict of interest related to the text presented.