Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2020)Volume 11, Issue 4
Background: The rate of Antibiotic Associated Diarrhoea (AAD) and Clostridium difficile associated diarrhoea (CDAD) have increased among hospitalized patients who are on long term of antibiotics therapy. Probiotics when combined with the usual treatment found to decrease the incidence rate of these cases. This systematic review evaluates the effectiveness of lactobacilli-based probiotics alone and multistrains of lactobacilli and bifido bacteria in AAD and CDAD.
Methods: Three databases: Google Scholar; PubMed and Cochrane central library were covered for randomized controlled trials from period 2005 till 2017. Studies were examined and filtered according to title/abstract and full text. The quality of included studies was assessed using Jadad scale.
Results: Eight articles were reviewed. Four articles investigated the use of various strains of lactobacilli-based probiotics among adult hospitalized patients who had already started antibiotics therapy. These showed that lactobacilli-based probiotics alone are effective in reducing both AAD and CDAD. Two of the studies used similar strains and similar doses, both resulted in significant reduction in diarrhoeal cases (OR 0.34; P=0.05) and (OR 0.667; P=0.067), respectively. While the other two studies showed less significance among hospitalized patients (OR 0.25 P=0.007, P< 0.001).
The other four articles investigated the use of lactobacilli probiotics in combination with bifodobacteria strains. Two studies were conducted on large number of patients using different doses of probiotics. Results showed these probiotics were not effective, AAD (P=0.71) and CDAD (P=0.35). One study showed the incidence of AAD was lower when higher dose of probiotics was used (P=0.08) while no difference in CDAD (P=0.02). The last study showed that patients who received Infloran(multistrainprobiotics) developed AAD and CDAD more than placebo group (P=0.246).
Conclusion: This review showed that lactobacilli-based probiotics alone are more effective in reducing AAD and CDAD than multistrain probiotics. More researches that involve large number of patients and use lactobacilli strains only are needed to imply its use in clinical settings.
Probiotics; CDAD; Multistrain; Diarrhoea
Antibiotic Associated Diarrhoea (AAD) is one of the common complications that is associated with antibiotics therapy. Its incidence ranges from 20 to 60% during periods of outbreaks in hospitals, while this rate is 13-29% during periods of endemics. The incidence is much lower in outpatient settings [1]. The antibiotics which are most responsible in causing AAD are: broad-spectrum penicillin; cephalosporins; cilindamycin and fluoroquinolones [2]. The mechanism by which antibiotics cause diarrhoea is by disruption of normal intestinal microflora and impairing their resistance, thus, resulting in alternating the carbohydrates, short chain fatty acids and bile acids metabolism [2]. It can occur few hours after commencement of antibiotics therapy or up to several months after stopping the treatment. AAD is a self-limiting condition if it is not caused by an infectious agent. However, this type of diarrhoea can be a result of Clostridium difficile infection (CDI). C. difficile can lead to serious and severe cases such as pseudomonous colitis or toxic megacolon if not treated properly [3].
One of the major concerns related to CDI is the emergence of new ribotype 027 strain. This strain is resistant to antibiotics treatment such as fluoroquinolones. It is found to be more prevalent in the UK [4] C. difficile associated diarrhoea (CDAD)accounts for 15- 25% of AAD cases. There are factors that increase the risk of AAD and CDAD such as patients’ age >65, prolonged hospital stays and long term of antibiotic use(Allen et al., 2013a). The conventional strategy for treating CDAD is administrating metronidazole and vancomycins antibiotics [5]. However, it has been suggested that microbial preparations in addition to the usual antibiotics treatment can reduce the incidence of AAD and CDAD among hospitalized patients [5]. These probiotics are thought to exert their effects through various mechanisms such as producing specific bacteriocins, competing with the infective pathogen for nutrients and binding sites or through modulating the immune response [1]. Lactobacilli, Bifidobacteria and S.boulardii are the most well-known probiotics used in clinical settings [3]. Although several systematic reviews have been performed to test the efficacy of various types of probiotics, S boulardii was the mostly investigated probiotics. Furthermore, reviews about the use of certain strains of lactobacilli have been performed. These studies came out with the result that probiotics are beneficial in reducing the incidence of AAD and CDAD but further studies with larger sample size are required to specify which strains of probiotics were responsible for the effectiveness [6]. This systematic review is considered the first to assess various strains of lactobacilli alone or combined with strains of bifidobacteria on hospitalized adult patients who are at risk of AAD and CDAD.
Literature search
The search covered three databases: Google scholar, Pubmed and Cochrane library. On Google scholar, the search terms in advanced search included: (lactobacillus and bifidobacterium and probiotics and prevention and treatment and Clostridium difficile and infection and diarrhea and antibiotic-associated diarrhea AAD and primary outcomes and secondary outcomes and hospitalised patients) from period of 2005 till 2017. The search on Pubmed included the terms: (((lactobacilli) and clostridium difficile prevention) and antibiotic-associated diarrhea) and ("2005"[Date - Publication]: "2017" [Date - Publication]). While in Cochranecentral library, the terms in advanced search included: (Lactobacilli and prevention and clostridium difficile and antibiotic-associated diarrhea) from period 2005-2017 and the search was limited by choosing trials only. The initial search included 444 articles, these were filtered according to title and abstract then examined according to full texts. Eight articles met the inclusion criteria and were included in this systematic review.
Inclusion criteria
The studies included are Randomised Control Trials (RCT) which are double blinded. These trials were performed on adult hospitalized patients who received antibiotics therapy for various indication smostly due to respiratory infection. The included trials must have assessed the use of various strains of lactobacilli- based probiotics of different doses either alone or combined with bifido bacteria strains. Patients who are included in the trials must not have 1- previous diagnosis of C. difficile infection nor have gone through gastrointestinal surgeries 2- no previous gastrointestinal diseases or basal diarrheal conditions 3-not immuno compromised. The included studies were only in English language. The reason for including double blinding trials specifically, is to avoid the bias in this systematic review by blinding the patients who received the probiotics and the individuals who measured the outcomes.
Exclusion criteria
Non-randomized, non-double blind trials and studies that performed on children or outpatients were excluded. Studies that assessed yeast probiotics such as S. boulardii or bifido bacteria alone were excluded to reduce the heterogeneity of the studies included. Trials that did not assess diarrhoea as an out come neither examining samples for C. difficile by culturing stool samples or detecting C. difficile toxins were excluded.
Baseline characteristics
Patients were >18 years old, hospitalized patients and have received antibiotics of various doses for different indications either single antibiotics or combined with other antibiotics of different administration routes. The active treatment of probiotics was given within 7 days of starting the antibiotics therapy. All patients were consented before they were recruited for the trials.
Study selection
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart. A study selection was based on removing duplicates and screening titles/abstracts and full texts (see Figure 1).
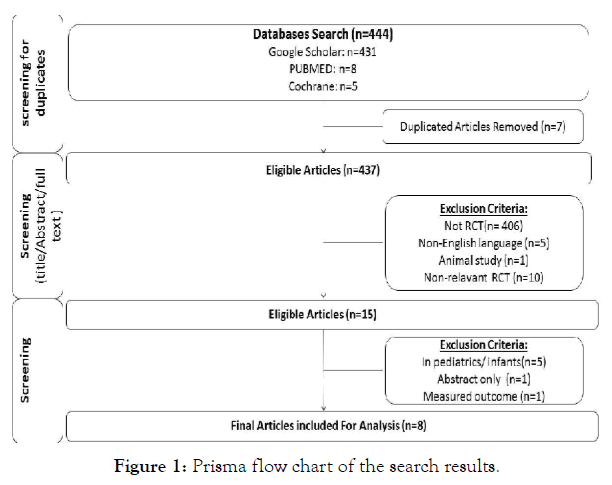
Figure 1: Prisma flow chart of the search results.
Quality ssessment
The included randomized controlled trials in the current study were assessed using Jadad scale. This scale consists of three assessment items which are: randomization method of the studies, the blinding of the studies and an account of all patients (withdrawals/ dropouts). The trial is given one point each if the randomisation, blinding and patients’ withdrawals/dropouts were mentioned. An additional point is assigned to the study if the method of randomisation and blinding were described appropriately. However, if the methods for randomisation and blinding were not appropriately described then one point is deducted. A score of 0 is considered of a low quality while a score of 5 is considered of a high quality.
Literature search
The total number of articles found in literature search in 3 databases were 444. A total of 435 articles were excluded for the various reasons such as using different type of intervention, different type of study or irrelevant. One article was excluded because only the abstract was published. Another study was excluded because the outcome measure did not meet the inclusion criteria of this systematic review. The selection process of studies resulted in including eight randomised controlled trials which have met the inclusion criteria.
Sample size of included trials ranged from 50 to 17,420 patients. These studies were performed in the UK, Canada, Korea, China and Thailand. Patients were given probiotics which contain various stains of lactobacilli either combined with bifidobacteria strains or not. These probiotics were given in form of fermented milk, yogurt or capsules along with the antibiotics therapy (see Table 1). Most of the trials were placebo controlled, except for one study, in which the placebo group was not mentioned [7]. Patients in all trials were followed up to test the efficacy of these probiotics by measuring the occurrence of AAD or CDAD among both treatment group and placebo group.
| Authors and references | Definition of diarrhea | Sample size of patients | Patients age | Type of probiotic used | Probiotic dose measured in colony forming units (CFM) |
Duration of treatment |
|---|---|---|---|---|---|---|
| (Hickson et al., 2007) | ≥ 2 liquid stools per day for 3 days or more | 135 patients |
Mean age 75 years | Yogurt containsL.casei+ S. thermophilus +L.bulgaricus |
1.0x 108 +1.0 x 108 +1.0 x107 /twice a day. |
48 hrs after commencement of antibiotics therapy and for one week after stopping antibiotics. |
| (Allen et al., 2013a) | ≥3 loose stools (Bristol score 5-7), or looser than normal according to patients during 24 hrs. |
17,420 patients |
≥ 65 years |
Capsules contain: two strains of L. acidophilus + two strains of bifidobacteria | 6 x 1010/ once capsule a day. |
21 days |
| (Wong et al., 2014) | ≥ 2 liquid stools (Bristol stool chart 5,6 or 7) per day for 3 days or more with quantity more than normal. |
164 patients |
≥ 18 years |
Drink contains L.casei Shirota |
6.5x 109 /once a day |
24 hrs after commencement of antibiotics therapy and one week after stopping antibiotics. |
| (Beausoleil et al., 2007) | ≥ 3 liquid stools in 24 hrs period. | 89 patients |
≥ 18 years |
Milk contains L. acidophilus CL1285 + L. Casei |
50 x 109 / once a day. |
48 hrs after commencement of antibiotics therapy and throughout the whole therapy period |
| (Sampalis et al., 2010) | One or more episodes of unformed or liquid stool in 24 hrs periods. |
216 patients. |
≥ 18 years. |
Fermented milk contains L. acidophilus CL1285 + L. casei (BIO K+ CL.1285) |
50x109 | 29- 40 days |
| (Ouwehand et al., 2014) | ≥ 3 liquid stools (Bristol stool chart 7) in 24 hrs periods and confirmed by 2 physicians. |
503 patients. |
30-70 years. |
Capsules contain L.acidophilus + L.paracasei + B.lactis Bi-07 and B.lactis Bi- 04 | Either low dose of 4.17 x 109 or high dose 1.70 x 1010 | 10-21 days (probiotic was taken till 7 days after stopping antibiotics) |
| (Iamharit and Harnsomburana, 2010) | ≥ 3 loose stools a day for at least 2 days or ≥ 5 times a day for a single day. |
50 patients |
20-75 years. |
Infloran Capsule which contains lyophilized live L.acidophilus+ B. bifidum |
1x109 + 1x109 |
14 days. |
| (Allen et al., 2013b) | ≥ 3 loose stools (Bristol stool scale 5-7) in 24 hrs period. |
17,420 | ≥ 65 years. |
Vegetarian capsulecontains: 2 strains of L. acidophilus + 2 strains of Bifidobacterium (lactis and bifidum) |
6 x1010 | 21 days. |
Table 1: Summary of 8 included articles in this systematic review.
Measuring risks of AAD and CDAD using Lactobacilli strains only
Four trials studied efficacy of various lactobacilli strains alone. These probiotics were not combined with other probiotic strains. The incidence of AAD among patients who received lactobailli probiotics in the four studies ranged from (3.9%-21.8%). While in placebo group, the incidence was higher (7.2%-54.9%). Furthermore, in two studies [6,7] no cases of CDAD were reported among patients who consumed probiotics, as compared with the control group in which several CDAD cases were reported. However, in the other two studies [3,8] only one case of CDAD was reported among the lactobacilli group (See Table 2). This indicates that lactobacilli strains alone are effective in reducing the incidence rate of AAD and CDAD among hospitalized patients.
| Study | Incidence of AAD | P-value | Incidence of CDAD |
P-value |
|---|---|---|---|---|
| (Hickson et al., 2007) |
12% | P=0.007 | 0% | P=0.001 |
| (Wong et al., 2014) |
17% | P< 0.001 | 0% | - |
| (Beausoleil et al., 2007) |
15.9 % | P=0.05 | 2.3 % | P=0.06 |
| (Sampalis et al., 2010) | 21.8 % | P=0.067 | 6.2% | P=0.645 |
Table 2: Incidence of AAD and CDAD among patients receiving Lactobacilli probiotics.
Measuring risks of AAD and CDAD using multistrains of Lactobacilli and Bifidobacteria.
The other four articles studied the effect of lactobacilli and bifido bacteria together. Their results showed that multistrains were not effective in reducing the incidence of AAD. Two studies (Allen et al., 2013a, Allen et al., 2013b), which were performed on similar sample size using same strains of lactobacilli and bifido bacteria but different doses of probiotics used in each. Both studies showed that the incidence of AAD ranged between (10.4%- 10.8%), respectively. The rates of AAD in both placebo groups were similar to probiotic groups which is of (10.4%) [2,9]. These results were supported by the third study [10] which showed the rates of AAD in patients consumed probiotics had increased (11.5%), compared to placebo group (0%). However, the fourth study [11] assessed the efficacy of different doses of probiotics. The results showed that incidence of AAD using high dose of probiotic preparations was (12.5%) compared to using low dose (19.6%), both doses decreased the rate of AAD compared to placebo group (24.6%).
On the other hand, the use of probiotics showed a trend in decreasing CDAD incidence among hospitalized patients in three studies. The results were similar in two studies (Allen et al., 2013a, Allen et al., 2013b), as the incidence of CDAD was only (0.8%), compared to placebo group (1.2%). While in the third study [11] the rate was similar in both groups who received various doses of microbial preparations which was (1.8%) compared to (4.8%) in placebo group. However, results of the fourth study [10] showed that one case of CDAD was reported inparticipants who received probiotics while there were no cases reported in placebo group (see Table 3).
| Study | Incidence of AAD |
P-value | Incidence of CDAD |
P-value |
|---|---|---|---|---|
| (Allen et al., 2013a) |
10.4% | P=0.71 | 0.8% | P=0.35 |
| (Ouwehand et al., 2014) | 18.9 % | P=0.02 | 2.8% | P=0.04 |
| (Iamharit and Harnsomburana, 2010) |
11.5% | P=0.246 | 3.8% | P=1.000 |
| (Allen et al., 2013b) |
10.8% | P=0.71 | 0.8% | P=0.35 |
Table 3: Incidence of AAD and CDAD among patients receiving multistrains probiotics.
Probiotics safety assessment
All articles have assessed the safety of microbial preparations used by patients. Generally, probiotics are safe and well tolerated. Participants in two studies [6,7] did not report any adverse events. While in four studies [3,10,11,8] participants reported non-serious adverse events which mostly were gastrointestinal related problems such as constipation, flatulence, nausea, bloating and abdominal distention.
Furthermore, there was no significant difference in the rate of these adverse events between the placebo groups and patients who received probiotics. Death cases were reported but these cases were not related to study preparations. However, two trials were performed on large sample size, showed that (19.7%) of the participants experienced one or more serious side effects, the most common side effects were related to respiratory, mediastinal, cardiac and gastric [2,9].
Quality assessment
The quality of the studies in this systematic review which were assessed using Jadad scale varied. Four of the eight studies are of score 5, which is considered of high quality as the randomisation, blinding methods were mentioned appropriately. Three other studies have a score between 3 -4, which is considered of a moderate quality. These studies did not include the randomisation and the blinding methods, thus, points were deducted. Only one article is of score 2 which is considered of poor quality as the blinding was not mentioned.
In this systematic review of eight randomized controlled trials, four studies used microbial preparations of various lactobacilli strains alone compared with placebo group. While the other four included trials that assessed the efficacy of probiotics that consist of lactobacilli and various strains of bifidobacteria compared to placebo groups. One study [7] which did not have placebo controlled group,the reason for this was explained in the study as using heat-treated lactobacilli might change the taste of probiotics, hence, affecting patients’ compliance to treatment.
Probiotics that contain lactobacilli strains only were found to be effective in reducing rates of both AAD and CDAD among adult hospitalized patients who had started antibiotics therapy for various reasons. However, the results varied by using probiotics that contain lactobacilli combined with bifido bacteria strains. These results showed these probiotics were not effective in reducing AAD but showed a trend in reducing the rate of CDAD among hospitalized patients except for one study in which one case of CDAD was reported among patients who received probiotics [10]. These findings are attributed to the fact that lactobacilli microbiota has the ability to survive through upper gastrointestinal tract and reach large intestine in an intact state. This has been seen by checking stool samples, which showed to contain stains of lactobacilli after being consumed by the patients [9]. While bifido bacteria quantity found to be reduced when antibiotics therapy is administered thus, the amount of probiotics is important to restore the intestinal microflora [12]. Additionally, this present study compared studies of various sample sizes. The studies that included small number of patients showed that probiotics were effective in treating AAD. This increased the risk of type II statistical errors, which means an already existing effect might be present but it has not been shown [12]. While a recent study performed by Allen and colleagues using a multistrain probiotics on a large sample size showed that probiotics were not effective and the preventive rates of AAD and CDAD were less than expected [2,9]. The reason that explains the difficulty in conducting trials using large sample size is the inability in obtaining large number of CDAD cases in order to validate and predict the effects of probiotics [13].
Regarding base-line characteristics of this study, patients age was set to be >18 years as the included trials showed a variety in patients’ age. Three trials included patients with mean age of more than 65 years old only. While other studies included patients, who are > 18 years old. This difference could have affected the studies’ outcome as C. difficile usually affect younger population, hence, affecting the studies generalizability [14].
While AAD incidence is found to occur more in older patients compared to younger patients (12.3% vs 6.6%) respectively, as demonstrated in one trial [11]. Probiotics are known to be strain and dose specific. However, the dose of probiotics was not specified in the inclusion criteria of this review. This is due to adose of probiotics more than 1010 has been suggested in order to produce the desired effects in preventing AAD among patients. However, there is a risk of increasing undesired adverse effects if the dose of probiotics is increased [7]. The high dose effect has been seen in the studies that used the suggested dose of probiotics, as more serious side effects were reported by patients [9]. This was further supported by another study that used a very low dose of multistrain spp. among hospitalized patients and showed that these strains were not effective in reducing AAD and CDAD [12]. Therefore, a moderate dose of probiotics would be sufficient to produce the required effect.
Although current guidelines do not recommend the use of probiotics, few reasons have been proposed to consider using probiotics specially lactobacilli spp. A study demonstrated that hospitalized patients might suffer from poor appetite, which can cause the patients to be undernourished, which increases the risk of AAD and CDAD. As a result, consuming probiotics and increasing the dietary fibre intake can enhance restoring gut normal flora and increase the amount of Short Chain Fatty Acids (SCFA). This helps in absorbing water and electrolytes from colon, hence, decreasing the risk of diarrhea [7].
Furthermore, a study has calculated the cost that is needed to treat one case of AAD and one case of CDAD which was £50 and £60, respectively [6]. Thus, the routine administration of probiotics can be of economic benefits by reducing the hospital stay and the treatment cost specially some cases require vancomycin administrations [6].
This present study findings stand in line with some previous systematic reviews and meta- analyses. These previous studies showed that probiotics specially the ones that contain lactobacilli spp. formula were effective. One meta-analysis assessed Lactobacilli rhamonsus GG in both adults and children. Results showed that it is preferred to take low dose oflactobacilli probiotics early along with antibiotics treatment before any changes occur in intestinal microbiota [15]. This was further supported by another systematic review which studied lactobacillus probiotics in preventing CDAD. the results were consistent with the current findings as lactobacillus can decrease the risk of C. difficile with relative risk reduction of 75% [13] Despite the positive outcomes, these two studies did not specify the population studied and that is considered as a limitation for these studies. Unlike the current study, which specified the use of probiotics on adult hospitalized patients.
The limitation of this study is excluding non-English studies. Additionally, some of the randomized trails might not have been located resulting in publication bias. Another limitation is the inclusion criteria of the present study led to limiting the number of studies that included. This review is also limited to assessing lactobacilli whether alone or in combination with bifido bacteria strains in adult hospitalized patients and not including paediatric age group or other probiotic strains. Furthermore, some studies that were published in abstract only, were excluded from this study to limit the risk of bias.
The current study assessed the efficacy of lactobacilli probiotics either alone or added to bifido bacterial strains. This is due to the incidence of AAD and CDAD have increased specially among hospitalized patients who are already on antibiotics therapy. Some of these patients may not show any response to the standard treatment of antibiotics or might become resistant to antibiotics. Since antibiotics are known to be the major factor to cause AAD and CDAD. Probiotics have been suggested as an adjunct treatment to prevent developing AAD and CDAD.
This study reviewed eight randomised trials. These trials studied lactobacilli spp. probiotics either alone or in combination with bifido bacterial strains on old hospitalized patients. Lactobacilli spp. alone seem to show some benefits of reducing the incidence of AAD and CDAD when given early during treatment period or along with antibiotics therapy. These benefits overweigh the clinical outcomes of lactobacilli when combined with bifido bacteria strains. Various doses of probiotics have been used in the eight trials, hence, the exact effective dose of lactobacilli is still to be determined as some studies showed that high dose can be more effective on patients, whilst most studies used lower doses yet proved to be effective. Only two trials have recruited large number of patients and showed that lactobacilli and bifido bacteria are not effective. Further researches with large sample size and using lactobacilli strains only need to be performed.
Citation: Al-Nasiri R (2020) Systematic Review: Lactobacilli and/or bifidobacteria in Preventing Antibiotics Associated Diarrhoea and C. Difficile Infection in Adults. J Drug Metab Toxicol. 11:251. doi: 10.35248/2157-7609.20.11.251
Received: 29-Jul-2020 Accepted: 12-Aug-2020 Published: 25-Sep-2020 , DOI: 10.35248/2157-7609.20.11.251
Copyright: © 2020 Al-Nasiri R. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.