Review Article - (2021)Volume 6, Issue 3
Although more than a year has passed since the COVID-19 outbreak, the pathogenesis of the disease has not yet been clearly explained. The way to develop effective vaccines and drugs against the disease depends on a clear elucidation of the pathogenesis. Therefore, the underlying mechanism of COVID-19 pathogenesis has been one of the most curious and studied topics since the beginning of the pandemic. Systemic organ involvement in COVID-19 cannot be explained by viral tropism mediated by ACE2 and TMPRSS2. Many chronic, inflammatory, and autoimmune diseases such as autism, dementia, and memory problems that emerged late after COVID-19 have made the pathogenesis of COVID-19 even more mysterious. Our studies and current literature show that the main problem in the pathogenesis of COVID-19 is retinol depletion and retinoid signal impairment. It is understood that many chronic diseases, serious clinical pictures, and residual diseases, which are called multisystem involvement in COVID-19, occur as a result of retinoid signal disorders. Due to the widespread distribution and intense activity of retinoid activity in the body, COVID-19 manifests itself with a very common and systemic involvement and a wide variety of symptoms and disease tables. Interestingly, organ involvement and disease severity of COVID-19 also parallels the intensity of retinoid activity in organs. Clarifying the pathogenesis of COVID-19 will enable us to develop effective strategies that will yield results regarding disease treatment, prophylaxis, and vaccination programs.
Vitamin A; Retinol; Retinoid; Retinoic acid; Retinoid Signaling; RIG-I; COVID-19; Organ Involvement; Cytokine; Autoimmunity
Although more than a year has passed since the COVID-19 pandemic, the pathogenesis of COVID-19 is still a mystery [1]. However, when the literature data and current research results are examined, it is seen that defective retinoic acid metabolism and retinoid signaling disorder are responsible for the destructive systemic effects in COVID-19. Here, the role of retinoic acid metabolism and retinoid signaling in the pathogenesis of COVID-19 and especially systemic organ involvement has been discussed, taking into account current literature information and COVID-19 research. The mechanisms presented here and that constitute evidence for retinoid signaling disorder and pathogenetic disorders in COVID-19 were evaluated in light of recent research data.
Retinol and retinoic acids are used in viral infections, Type I interferon synthesis, and suppression of inflammation. In [2-4] COVID-19, retinol is used too much and consumed quickly due to the extremely large viral genome. As a result, retinoid signaling disorder occurs in COVID-19 due to regular ATRA levels. Here, complete depletion of retinol is not necessary for the development of retinoid signaling disorder, as retinol levels fall below normal serum levels are sufficient to disrupt retinoid signaling [5]. Although the destructive systemic effects caused by the retinoid signaling disorder in COVID-19 are tried to be explained with the viral tropism provided through the ACE2 and TMPRSS2 receptors, the pathogenetic mechanism operating through viral tropism is insufficient to explain the systemic disorders here [6,7].
It has been determined in many previous studies that vitamin A deficiency reduces host resistance against viral infections and vitamin A levels decrease during viral infections [8,9]. Retinol levels also decrease in COVID-19, which causes a severe clinical picture and systemic organ damage. Retinol is depleted rapidly in COVID-19 due to the massive SARS-CoV-2 genome (30 kB) and the heavy catabolic process. In COVID-19, severe illnesses, including some mild symptoms and clinical findings, and post- COVID syndromes, also known as residual diseases, seen in the post-COVID period also occur as a result of retinoid signaling disorder.
In our recently concluded clinical study with preprint pressure in medRxiv, serum retinol levels were found to be significantly lower in severe COVID-19 patients compared to the control group [10]. In COVID-19, retinoid signaling defect is the main pathogenetic disorder, and immune system dysregulation, Type I interferon synthesis defect, severe inflammatory process, and destructive systemic effects are caused by retinoid signaling defect [10]. The rehabilitation of retinoid signaling can prevent the severe COVID-19 picture, as well as chronic, autoimmune, and inflammatory diseases that occur late after COVID-19 and are also defined as post-COVID syndromes.
Vitamin A is probably the most essential and functional vitamin in the human body, as it is absolutely essential for both maintaining organogenesis and maintaining biological functions from the embryonic stage to adulthood [11]. Essentially, vitamin A and its metabolites, retinoic acids, function as hormones in terms of structure and function. When it was first discovered, vitamin A was named as hormone A and growth factor because of its vital functions, and later this vitamin continued to be called an antiinfectious vitamin due to its protective role against infections [11]. The World Health Organization added vitamin A to its prevention programs during measles epidemics in the 1950s and achieveds successful results. Impressive results have been obtained from supportive therapies with vitamin A in [9,12] AIDS patients [13]. In addition, vitamin A and its derivatives have been used as vaccine adjuvants for many years [14].
The biochemical conversion and activity of retinoids or carotenoids to retinoic acids are necessary for the regulation and maintenance of a wide variety of and important biological functions such as growth, development, proliferation, differentiation, and apoptosis, both in the intrauterine period and in the adult period [15]. However, irregular retinoid signaling can contribute to serious illness. Retinoids fulfill these effects through nuclear retinoic acid receptors through genomic and non-genomic mechanisms [16-18]. Retinoid metabolism is largely achieved by endogenous regulation of retinoic acids through the cytochrome oxidase P450 enzyme system, while retinoid signaling is achieved through retinoic acid receptors. Vitamin A is provided by All-Trans Retinoic Acid (ATRA) and 9-Cis retinoic acid (9-CisRA), which are active derivatives [19,20].
The synthesis of ATRA, the most active and functional retinol derivative, which is largely responsible for retinoid signaling, is performed by a large number of functional cells in the host [15-19]. ATRA synthesis, plasmocidic dendritic cells, primarily monocyte, macrophage, and dendritic cells from immune system cells, Gut-Associated Lymphoid Tissue cells (GALT) in the intestine, plasmocidic dendritic cells in the lung, alveolar macrophages, type II pneumocytes and progenitor and support cells in the olfactorys and taste epithelium, oligodendrocytes in the brain, astrocytes, and swan cells in axon sheaths. In all these cells and related organ systems, RALDH enzymes, CYP450 enzymes, and retinoic acid receptors also display a very intense positioning and activity for retinoic acid synthesis, metabolism, and functions [17,19,20].
The pleitropic genomic effects of vitamin A are mainly mediated by the expression of target genes via several nuclear receptors. Retinoids bind to nuclear receptors called Retinoic Acid Receptors (RAR) and Retinoid X Receptors (RXR), modulating transcription factors and a series of co-activators and repressors that act as nucleus DNA binding and regulators, regulating physiological outputs [21]. Active metabolites of retinol, ATRA, and 9-CisRA, bind to Retinoic Acid Response Elements (RARE) and regulate gene expression by activating transcription complexes with retinoic acid receptors. With this genomic signaling mechanism, genes products ultimately emerge [21,22].
Retinoid signaling also includes non-transcriptional non-genomic effects in the cytoplasmic domain, in the form of activation of kinase cascades by phosphorylation of various enzymes. Most of these effects are provided by the glutamate subunit 1 receptor (GluR1), which is synthesized by cytoplasmic RAR activity. The GluR1 receptor is mainly responsible for providing neuronal plasticity and synaptic transmission in the nervous system [23]. While the retinoid signaling mechanism operating through retinoic acid receptors is responsible for the formation and development of organs in the embryonic period, it is responsible for the maintenance of biological functions in adulthood. Disruption of retinoid signaling in the embryonic period causes serious congenital malformations, and in adulthood, it causes many serious diseases, especially immune system depression and inflammatory pathogenesis [24].
Viruses generally show an affinity for certain cells and tissues of the host. Some viruses are even classified according to this tissue and cell affinity (tropism). Like neurotrophic, hepatotropic, enterotropic viruses. Tissue damage in the host usually occurs in the organs of the virus that are related to this cell and tissue tropism [25]. COVID-19 also has a cell and tissue tropism provided through ACE2 and TMPRSS2 receptors. However, this tropism is limited to a few organ involvements and is mainly associated with the respiratory and gastrointestinal system involvement of SARS-CoV- 2 [26]. The multi-organ involvement and destructive systemic effects in COVID-19, which cannot be explained by the affinity of SARS-CoV-2 with ACE2 and TMPRSS2, can be clearly explained by retinol depletion and retinoid signaling disorder [27-29].
This systemic involvement in COVID-19, which cannot be explained by viral tropism, has made COVID-19 pathogenesis a mystery. During the pandemic, most researchers have spent a lot of time in pursuit of the viral tropism mechanism to solve the pathogenesis of COVID-19. Essentially, the main difference that distinguishes COVID-19 pathogenesis from other virus infections is the excessive size of the SARS-CoV-2 genome and the retinol depletion and retinoid signaling defect it causes [30]. While the genome size of other RNA viruses is 8-10 kb, the genome size of SARS-CoV-2 is approximately 30 kb. This makes SARS-CoV-2 the virus with the largest genome of all RNA viruses [28,30,31]. Because of this genome size, large amounts of RNA particles are released from viruses that are broken down by host immune system cells during infection. Because of these scattered RNA ends, RIG-I and TLR receptors are overstimulated and consume retinoic acids [32,33]. Retinoic acids are used herein the synthesis of Type I interferon via RIG-I and TLR and in the suppression of inflammation. Retinoid signaling disorder develops in COVID-19 due to depletion of retinol and retinoic acids.
TLR receptors on cell surfaces are responsible for the recognition of RNA viruses in the early stage of viral infection, and this function is undertaken by the RIG-I receptor in the cytosol as the infection progresses and the virus enters the cell [32,33]. After this stage, the immune system cascade continues through RIG-I. RIG-I is the main cytosolic receptor that recognizes intracellular RNA viruses and is responsible for type I interferon synthesis, and as the name suggests, it is synthesized due to retinoic acids [33,34]. With the depletion of retinol and the development of retinoid signaling disorder, the RIG of the congenital immune system where Type I interferon synthesis is provided. By collapsing the -I pathway, the UPS/NFкB system, which simultaneously operates through the TLR receptors of the adaptive immune system and causes excessive cytokine release, comes into play [35]. This pathogenesis mechanism, which we proposed for the pathogenesis of COVID-19 for the first time in the study titled “Endogenous Retinoic Acid Theory and Retinoic Acid Depletion Syndrome”, is fully compatible with the biphasic immune pathogenesis in COVID-19, on which the researchers agree, and is accepted by researchers [35].
In COVID-19, decreasing ATRA levels with the use of retinol by host defense cells disrupts the delicate balance between regular T cells of the adaptive immune system and pathogenic Th17 cells, causing dysregulation in the immune system. In this way, the balance between the congenital and adaptive components of the immune system is broken, resulting in biphasic immune pathogenesis resulting in the collapse of the congenital immune system and hyperactivation of the adaptive immune system [35]. With the normal serum levels and efficiency of ATRA, self-tolerance is provided by the transformation of naive T cells into regular T cells in the host. . With this mechanism, tolerance develops against both the intestinal microbiota and the host's self-antigens. Here TGFβ strengthens the self-tolerance effect of ATRA. Th17 cell activity and inflammatory cytokine release are also inhibited with normal ATRA concentration and regular T cells (Figure 1) [36,37].
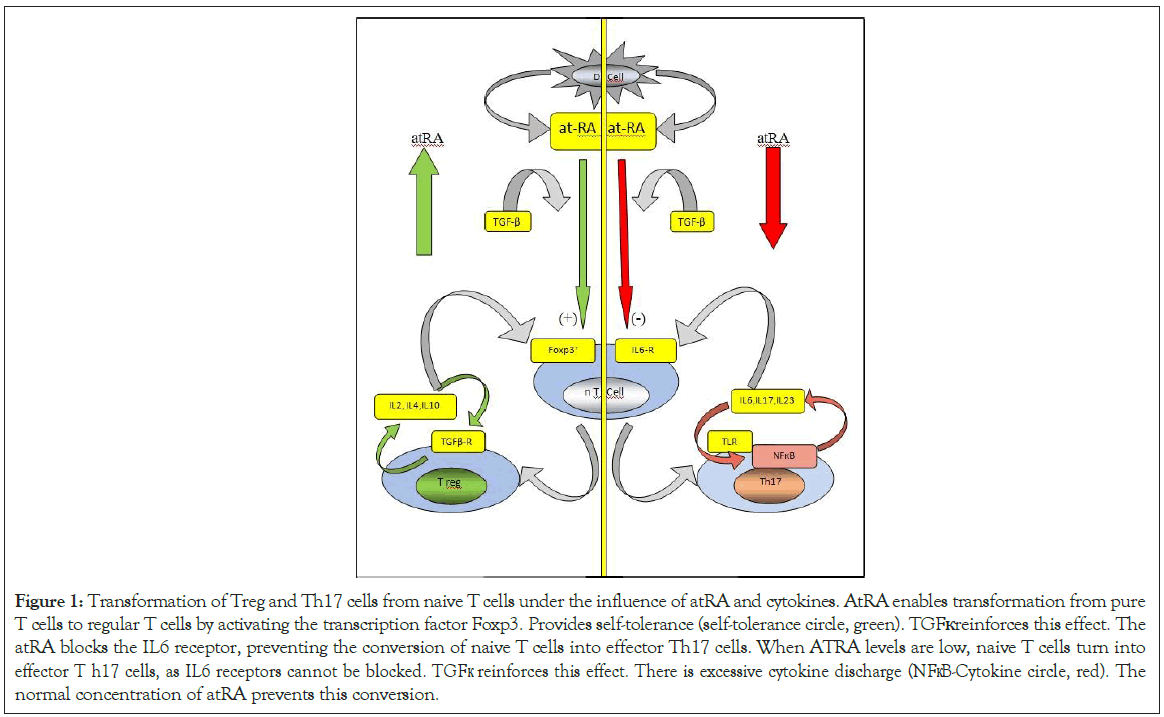
Figure 1: Transformation of Treg and Th17 cells from naive T cells under the influence of atRA and cytokines. AtRA enables transformation from pure T cells to regular T cells by activating the transcription factor Foxp3. Provides self-tolerance (self-tolerance circle, green). TGFк reinforces this effect. The atRA blocks the IL6 receptor, preventing the conversion of naive T cells into effector Th17 cells. When ATRA levels are low, naive T cells turn into effector T h17 cells, as IL6 receptors cannot be blocked. TGFк reinforces this effect. There is excessive cytokine discharge (NFкB-Cytokine circle, red). The normal concentration of atRA prevents this conversion.
However, with the decrease of ATRA levels, the conversion from naïve T cells to regular T cells decreases, and the transformation from naïve T cells to pathological Th17 cells responsible for pathological cytokine discharge increases [36-38]. As a result of this shaft shift in the immune system, excessive cytokine release (cytokine storm) occurs with increased NFкB activation in monocyte, macrophage, and dendritic cells of the adaptive immune system, especially reactive Th17 cells [38]. The main reason for this sudden and unexpected phase change between the congenital immune system and the adaptive immune system in COVID-19 is the decrease in retinol and ATRA levels. While regular T cell activity decreases due to the decrease in ATRA levels, effector Th17 cell activity, which is responsible for excessive cytokine release of the adaptive immune system, also increases.
Meanwhile, genomic DNA and RNA fragments and mitochondrial DNA fragments emanating from host cells as a result of apoptosis and cell lysis with RNA fragments released from viruses broken down by host cells continue to activate the inflammatory NFкB cascade through TLR receptors. This continuous and intense stimulation of TLR receptors causes excessive stimulation of adaptive immune system cells [39,40], excessive activity of NFкB, and excessive cytokine discharge. Due to this excessive cytokine discharge, devastating systemic effects occur [40].
Another factor that causes cytokine discharge out of control is the bilateral synergistic interaction between NFкB and cytokines [41]. ATRA controls NFкB activity and cytokine discharge by inhibiting degradation in the UPS system at normal and high concentrations [42,43]. However, with the decrease in ATRA levels, the conversion from naive T cells to Th17 cells increases, and the UPS / NFкB system gain activity [44-46]. The key molecule in the regulation of inflammatory mechanisms is ATRA, and the congenital immune system collapses with the decrease in ATRA levels and the adaptive immune system gains excessive activity. Here, the role of cytokines is secondary, with cytokines having a synergistic effect on their synthesis and secretion due to their bilateral interactions with NFкB. Cytokines are here responsible for aggregating their secretion and synthesis as secondary mediators and for systemic inflammatory activity and end-organ damage [42,43,47]. Due to this synergistic and bilateral interaction between NFкB and cytokines, a vicious circle occurs in inflammatory mechanisms. This vicious circle causes an uncontrolled increase in inflammatory mediator synthesis and discharge (NFкB-Cytokine circle) [47].
In order to trigger the inflammatory mechanism, there is no need to completely deplete retinol and ATRA in the host. If retinol levels fall below normal serum levels, it is sufficient to trigger inflammation [16,48,49]. This is due to the dose-dependent bimodal effect of vitamin A on inflammatory mechanisms. While vitamin A can inhibit NFкB activation and cytokine discharge with its degradation inhibition effect on the UPS system at normal serum levels, it cannot show this effect at low doses. As the pressure on the UPS system is removed with the decrease of 50 ATRA levels, UPS degradation valves are opened and IкB is detached from NFкB and NFкB is activated [43,47]. With the increased NFкB activity, the discharge of cytokines and other inflammatory mediators begins.
This bimodal mode of action of vitamin A has caused many researchers to confuse. In some studies, the increase in inflammation has been attributed to the presence of ATRA in the environment. It has been claimed that ATRA provides self-tolerance by acting as a mucosal adjuvant under stable conditions, but increases inflammation under inflammatory conditions. Also, it promotes effector T cell responses during RA, infection, or autoimmune diseases. Therefore, RA plays a role in immune homeostasis at a steady-state but activates pathogenic T cells under inflammatory conditions. These understandings of vitamin A and inflammatory mechanisms have been repeatedly emphasized in numerous studies over the years.
However, the presence of ATRA is indispensable for the vital functions of the cell and keeping inflammation under control. Vitamin A must be present in serum at normal (physiological) or therapeutic concentrations to suppress the excessive inflammatory response [50]. It is not the presence of ATRA that increases inflammation. The bilateral synergistic action of cytokines with NFкB aggravates inflammatory processes. The ATRA loses its effect of suppressing inflammation in low doses and plays a role in triggering inflammatory processes. Therefore, it should be essential to keep ATRA concentrations within the therapeutic limits in studies related to inflammation [51,52].
The immune system changes in COVID-19 exactly coincide with the immune system suppression that occurs in vitamin A deficiency. In fact, the biphasic immune pathogenesis in COVID-19 appears to be a clinical reflection of the bimodal mode of action of vitamin A [53-56]. Due to the widespread distribution and intensive activity of retinoid signaling in organ systems, COVID-19 affects almost all organ systems. Therefore, COVID-19 presents very common and diverse symptoms, clinical findings, and multiple organ involvement [57]. Retinoid signal impairment in COVID-19 is also responsible for severe clinical pictures, multiple organ injuries, and chronic post-COVID syndromes that occur in the late period, which are called "Residual Diseases" [58-60].
While COVID-19 passes with very mild symptoms without any symptoms in some individuals, it results in very severe clinical pictures and death in some individuals. Although some comorbid conditions and diseases that predispose to severe clinical picture have been identified, the main reason for this difference between individuals related to the course of COVID-19 is due to the state of retinol storage and the different activity (polymorphism) of CYP450 enzymes responsible for retinoic acid metabolism [61,62]. Malnutrition, chronic liver and lung diseases, chronic kidney disease, obesity, hepatosteatosis, chronic autoimmune, inflammatory and rheumatic diseases, malignancies, organ transplants, and old age are conditions that weaken and decrease retinol reserves. Since retinol stores are weak in these diseases, the immune defense against SARS-CoV-2 is also weak. Despite all these comorbid diseases and the weakening of the immune system caused by senility, the main reason for the severe and high mortality of COVID-19 in the elderly population is the low vitamin A reserves in the elderly group and the defective vitamin A metabolism. Although there is an understanding that vitamin A deficiency is specific to underdeveloped countries, childhood age groups, and pregnant women, vitamin A screenings conducted in China and Brazil in recent years revealed that vitamin A deficiency in the elderly population over 60 years of age living in big cities is not underestimated [63-66]. Even these rates are high, which will cause COVID-19 to be more common and more severe in large cities and the elderly population.
The protective effect of vitamin A against infections has been clearly demonstrated in previous studies. With the COVID-19 pandemic process, the immune-enhancing effectiveness of vitamin A as an immune modulator, anti-infectious, anti-inflammatory, and a good adjuvant should be evaluated. Considering that vitamin A deficiency creates susceptibility to infection by weakening the immune system and is necessary for the development of permanent immune response, detection, and monitoring of retinol levels before and during the course of the disease may be useful for good epidemic management [67-70].
Low vitamin A in COVID-19 causes Types I interferon synthesis defect and the inflammatory process out of control. Therefore, monitoring serum retinol levels during the course of the disease in COVID-19 and reinforcing the deficiency when necessary may prevent the development of a severe clinical picture. Vitamin A is severely reduced during infections, especially in infections that are devastating to retinol metabolisms, such as measles, respiratory syncytial virus, Ebola virus, Human Immun Deficiency Virus, and COVID-19. It is not tolerable by the host due to the severe clinical pictures and immunodeficiency caused by vitamin A deficiency and acute depletion. Therefore, it is mandatory to supplement retinol or restore retinoid signaling with retinoic acids in patients with decreased retinol levels.
The immune response of the host against SARS-CoV-2 in the early stage of COVID-19; the state of the retinol depot is closely related to retinoic acid endogenous levels and the early Type I interferon synthesis they provide. Good liver retinol reserve and especially serum levels of retinoic acids at the therapeutic range may be an effective strategy against SARS-CoV-2 in host defense. Vitamin A and carotenoids can work for COVID-19 prophylaxis as well as for many other infections such as Measles and HIV. Early depletion of retinol depot in COVID-19 is effective in aggravating the clinical picture. In addition, well-planned controlled clinical studies are needed for the inclusion of vitamin A or retinoic acid derivatives into the treatment protocols of COVID-19.
With epidemiological studies to be conducted, determining vitamin A deficiency on a social scale and feeding the society with foods rich in retinoid and carotenoids, giving prophylactic retinol supplements when necessary, as in previous measles epidemics, will also ensure the protection of the society from many other viral infections, especially COVID-19. Another important issue is related to vaccination programs. By checking vitamin A levels before vaccination, the effectiveness of vaccines can also be increased by giving vitamin A supplements to those with vitamin A deficiency. This is a kind of adjuvant prophylactic application. During the clinical course of COVID-19, the decrease in retinol levels and the predisposition of vitamin A deficiency to severe disease make prophylactic and adjuvant applications in COVID-19 even more important.
Lastly, this hypothesis, presented in this study and supported by current literature information, is a new and different view on the pathogenesis of COVID-19 and is used for the treatment and prophylaxis of COVID-19 as well as many other autoimmune, chronic, degenerative, and inflammatory diseases will be a guide. The hypothesis and suggestions presented here are expected to lead to a paradigm shift regarding infections, microbiota studies, autoimmune pathogenesis, and inflammatory mechanisms. This approach to inflammatory mechanisms will bring a new perspective to severe infections, sepsis, cytokine storm, autoimmune diseases, allograft reactions, vaccine and adjuvant molecules, degenerative neurological diseases, and cancer physiopathology. Therefore, the COVID-19 pandemic will be a milestone for this new approach to the immune system and inflammatory mechanisms, as in many areas.
Citation: Sarohan AR (2021) Systemic Organ Involvement and Retinoid Signaling Disorder in COVID-19. Immunogenet Open Access. 6:145.
Received: 10-Mar-2021 Accepted: 24-Mar-2021 Published: 31-Mar-2021 , DOI: 10.35248/igoa.21.6.145
Copyright: © 2021 Sarohan AR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.