Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Research Article - (2022)Volume 11, Issue 4
Controlled release drug delivery systems are gaining importance in the recent years for their clinical benefits which are not obtained from conventional oral drug delivery. Natural polysaccharides are the choice of materials among the hydrophilic polymers used, because they are nontoxic, less expensive, and widely available. One such polysaccharide is Tamarind Seeds Polysaccharides (TSP) which isolated from the tamarind kernel powder. This study is aimed to formulate tablet of diclofenac sodium with tamarind seeds polysaccharides by wet granulation technique, evaluate drug release characteristics, and compare it with the release profile of conventional tablets and that formulated with synthetic polymer Hydroxy Propyl Methyl Cellulose (HPMC). The results of the study was indicated that the drug release was extended over a period of 8 hours with tablets formulated with TSP and HPMC with a percentages 78.63%, 74.68% respectively. This study was put attention towards natural excipients which attribute to their safety, freely available, and cheap compared to synthetic one. TSP tablets stability and in vivo drug release should be further studied.
TSP; Diclofenac sodium tablets; Hydroxy propyl methyl cellulose; Drug release
In long-term therapy for the treatment of chronic disease conditions using NSAIDs, antihypertensive, anti-asthmatic and antipyretic drugs, conventional formulations are required to be administered in multiple doses which have several disadvantages [1]. Controlled Release (CR) tablet formulations are recommended for therapeutic benefits because they lead to patient compliance, maintain constant drug release concentration, reduce dose and side effects, and increase the safety margin for high-potency drug [2]. The use of natural polymers and their semi-synthetic derivative in drug delivery continues to be an area of active research [3]. The plant based polymers have been studied for their application in different pharmaceutical dosage forms like matrix controlled system, film coating agents, buccal films, microspheres, nanoparticles, viscous liquid formulations like ophthalmic solutions, suspensions, implants and their applicability and efficacy has been proven [4]. These have also been utilized as viscosity enhancers, stabilizers, disintegrants, solubilisers, emulsifiers, suspending agents, gelling agents, bioadhesives and binders in the above mentioned dosage form [5]. These polymers such as natural gums and mucilage are preferred over semisynthetic and synthetic excipients because of they are biocompatible, safe for human consumption, cheap, available and less toxic [6]. Drug release retarding polymers are the key representative in matrix systems [7]. Matrix systems are usually classified into three main groups: Hydrophilic, hydrophobic and plastic. Hydrophilic polymers are the most suitable for retarding drug release and also sustained drug delivery. There are various numbers of natural polymers which have been investigated as sustained release agent [8]. Tamarind Seeds Polysaccharides (TSP) is one of these polymers extracted from the kernel of seeds of Tamarindus indica, Family Leguminosae [9,10]. Therefore, this study aimed to formulate TSP tablets and evaluate their properties compared to HPMC and conventional tablets.
Plant materials
One kilogram of tamarind fruit was purchased from Wad Medani market, Gezira state, Sudan. The studied plant part was identified and authenticated by Medicinal and Aromatic Plants Research Centre, Faculty of Pharmacy, University of Gezira and Agricultural Research Corporation (ARC), Wad Medani, Sudan.
Extraction of tamarind seeds polysaccharides (TSP)
The whole fruits were soaked in water for twelve hours to remove the pulp. 600 g of tamarind seeds were obtained then soaked in distilled water and boiled for five hours to remove the outer dark layer. After removing the outer dark layer, about three liters of distilled water was added to the inner white portion and boiled with constant stirring in order to obtain the slurry. After that the solution was concentrated on a water bath at 60ºC to reduce the volume to one-third of its initial volume, the solution of one liter was cooled and poured into three liters of acetone with continuous stirring, then was allowed to settle down in the refrigerator for 2 days. After that the precipitate was filtered and finally dried using freeze drier for 24 hours [9].
Preparation diclofenac sodium tablets using TSP
200 mg TSP was mixed with 100 mg of Diclofenac Sodium and 193 mg of lactose (per tablet). Then the powders were granulated with isoproplyalcohol/water (3:1). The wet mass obtained were dried in oven at 40°C for 30 minutes. The dried granules were subjected to dry screening passing through mesh no. 14, blended with a mixture talc and magnesium stearate.
Preparation of diclofenac sodium tablets with HPMC
200 mg Hydroxypropyl Methyl Cellulose (HPMC) was mixed with 100 mg of diclofenac sodium and 193 mg of Carboxy Methyl Cellulose (CMC) per tablet. Then the powder was granulated using distilled water. The wet mass obtained was dried in oven at 40°C for 30 minutes. The dried granules were subjected to dry screening, passing through mesh no. 14, blended with a mixture talc and magnesium stearate.
Preparation of conventional tablet of diclofenac sodium
100 mg of sodium starch glycolate, 80 mg of maize starch and 188 mg of lactose was added to 100 mg of Diclofenac Sodium (per tablet) and mixed together. Then the powder was granulated using distilled water. The wet mas obtained were dried in oven at 40ºC for 45 minutes. The dried granules were subjected to dry screening passing through mesh no. 14, blended with magnesium stearate.
Tablets compression
Each 500 mg of granulated powder with HPMC and with TSP were weighed individually using electrical balance, then filled manually to the die of a single punch tableting machine and compressed. This process used to produce 20 tablets for each HPMC and TSP and 15 tablets of conventional Diclofenac Sodium (each tablet contain 470 mg).
In vitro release study
Construction of calibration curve: A stock solution with a concentration of 100 µg/ml was prepared by weighing out 25 mg of the diclofenac Sodium, transferring to a 250 mL volumetric flask and diluting to the mark with distilled water. Through appropriate serial dilutions, 5 calibration solutions were prepared as shown in Table 1.
| Concentration µg/ml | Dilution required |
|---|---|
| 4 | 1 ml diluted to 25 ml |
| 8 | 2 ml diluted to 25 ml |
| 12 | 3 ml diluted to 25 ml |
| 16 | 4 ml diluted to 25 ml |
| 20 | 5 ml diluted to 25 ml |
Table 1: Serial dilutions of stock solution.
The actual concentrations of the calibration solutions were calculated. The absorbance of each calibration solution was measured at 276 nm a plot of absorbance vs. actual concentration was produced. A linear regression analysis was carried out to determine the equation of the relationship between absorbance and concentration (i.e., y=mx+c).
Preparation of stimulated gastric fluid (pH 1.2)
According to British pharmacopeia (2009), exactly 59.5 ml of concentrated hydrochloric acid HCL (0.1 M) was diluted to 7000 ml with distilled water, to obtained final volume of pH 1.2 [11]. The pH was measured using pH meter (JENWAY)®.
Preparation of simulated intestinal fluid phosphate buffer, pH (6.8)
Seven litters of stimulated gastric fluid were prepared by mixing 827.25 ml of (0.2 M) potassium dihydrogen orthophosphate with the quantities of 1750 ml of (0.2 M) sodium hydroxide and then were diluted up to 7 L with distilled water [11]. The pH was measured using PH meter (JENWAY)®.
In vitro dissolution test
In vitro drug release studies were carried out using dissolution apparatus type 1, Rotatory basket method [12]. The 6 beakers was filled with 900 ml of acidic media. Temperature was adjusted at 37 ± 0.5 °C, employing a basket stirrer at 50 rpm. Samples of 5 ml of each beaker were withdrawn at different time intervals (5, 10, 15, 30, 60, 90, 120 minutes) over a period of 2 h. Each sample withdrawn was replaced with an equal amount of fresh dissolution medium. Absorbance of each sample was measured using UV-spectrophotometer at wave length 276 nm. After 2 hours the acidic media was withdraw carefully and replaced by 900 ml of alkaline media. Samples of 5 ml of each beaker were withdrawn at different time intervals (1, 2, 3, 4, 5, 6, 7, and 8 h). Each sample withdrawn was replaced with an equal amount of fresh dissolution medium. Absorbance of each sample was measured using UV-spectrophotometer at wave length 276 nm.
Quality control analysis of formulated tablets
General appearance: The general appearance of tablets, its visual identity and overall elegance was essential for patient acceptance. It was included tablet size, shape, surface texture, color as well as presence or absence of an odor or taste.
Weight variation test: Ten tablets were selected randomly and weighted individually on accurate electronic balance, and then the total average weight was calculated. Not more than two of the individual weights of tablets were deviated from the average weight.
Tablet thickness calculation: Tablet thickness is an important characteristic in reproducing appearance and also in counting by using filling equipment. Ten bilayer tablets were taken and their thicknesses were measured using Caliper Vernier apparatus.
Friability test: The friability of the tablets was determined using friabilator. It is expressed in percentage (%). Ten tablets were weight collectively and placed in the chamber of the friabilator. In the friabilator the tablets were expressed to rolling resulting free fall of tablets within the chamber of the friabilator. It was rotated at a rate of 25 rpm. After 100 rotations (4 min), the tablets were taken out. From the friabilator and intact tablets were again weighted collectively. The percent friability was determined using the following formula [13]
Friability=W1-W2/W1 × 100
Where W1: Weight of the tablets before test.
W2: Weight of the tablet before test.
Data analysis: All the obtained data were expressed as means ± Standard Error of Means (SE) and Analyzed Using Analysis of Variance (ANOVA). Comparisons with the control groups were made using One-way ANOVA. The level of statistical significance was set at p<0.05.
Extraction of TSP
TSP (11 g) of was obtained from the extraction of 600 g tamarind seed. The isolated TSP powder was sparingly soluble in cold water and insoluble in ethanol and acetone. It was soluble in hot water forming colloidal solution.
Formulation of tablets
General appearance of conventional Diclofenac sodium tablets were good, have soft texture, clear color and normal size. Tablets formulated with TSP also have soft texture and normal size but the color was not clear. Diclofenac sodium tablets formulated with HPMC have porous texture, normal size and clear color (Figure 1).
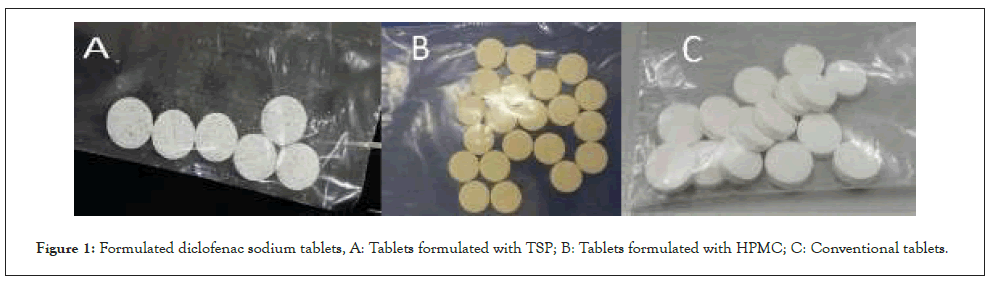
Figure 1: Formulated diclofenac sodium tablets, A: Tablets formulated with TSP; B: Tablets formulated with HPMC; C: Conventional tablets.
Quality control analysis of formulated tablets
The calibration curve was found to be linear in range of 4-20 µg/ml with a regression coefficient of 0.9989 and a linear line equation of Y=0.0312 x as shown in Figure 2.
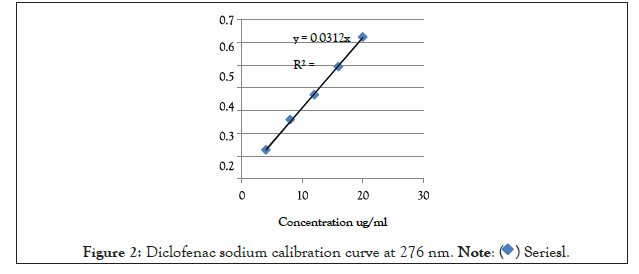
Figure 2: Diclofenac sodium calibration curve at 276 nm.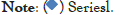
Dissolution test
The results of in vitro study were indicated that the percent of diclofenac sodium released after 8 hours from the HPMC and TSP were 74.62%, 78.63% respectively (Table 2 and Figure 3). After 2 hours the release of conventional Diclofenac Sodium tablet was 84.16%. The previous study was indicated that 300 mg of TSP per tablet is the optimum concentration for retardation effect with a release of 98% after 12 hours [14], but in this study 200 mg of TSP per tablet was used, produce a release of 78.63% after 8 hours. Also other study on TSP sustained release behavior of theophylline and indomethacin was indicated that the rate of release was in the decreasing up to 10 hours with 50% [15].
| Time (h) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| TSP | 15.59% | 26.27% | 34.11% | 42.97% | 52.59% | 65.23% ± | 78.63% |
| ± 0.03 | ± 0.32 | ± 0.32 | ± 0.32 | ± 0.68 | 0.69 | ± 0.76 | |
| HPMC | 17.89% | 25.52% | 33.33% | 41.16% | 49.44% | 64.2% | 74.62% |
| ± 1.22 | ± 0.58 | ± 0.63 | ± 0.40 | ± 0.30 | ± 0.30 | ± 0.30 |
Table 2: The dissolution profile for diclofenac sodium tablet using TSP and HPMC.
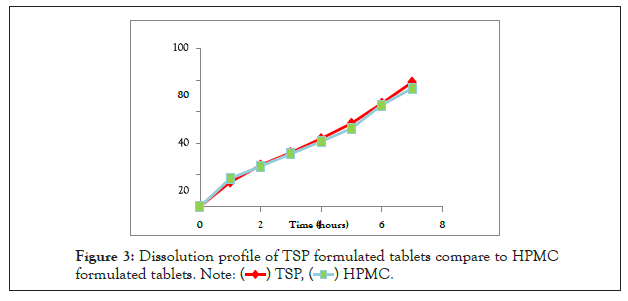
Figure 3: Dissolution profile of TSP formulated tablets compare to HPMC
formulated tablets.
The release of the diclofenac sodium from the tablets formulated with TSP giving release of 10.73% in acidic media, while the release of conventional diclofenac sodium tablets or immediate release tablets (I.M) is 84.16% after two hours, which indicates that the release from TSP is prominently occur in alkaline media (Table 3 and Figure 4). Therefore, TSP prevents the release of the drug in gastric environment because acidic pH minimizes the repulsive forces between negatively charged ions, while the basic environment causes deprotonation of carboxylic acid which causes electrostatic repulsion in between the negatively ions [16].
| Time (min) | 5 | 10 | 15 | 30 | 45 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|---|---|
| TSP | 0.14% | 0.39% | 0.62% | 3.56% | 4.07% | 4.69% | 7.73% | 10.73% |
| I.M | 13.67% | 24.96% | 34.67% | 46.11% | 59.99% | 66.59% | 74.16% | 84.16% |
Table 3: The dissolution profile for diclofenac sodium tablet using TSP compared with conventional diclofenac sodium tablet.
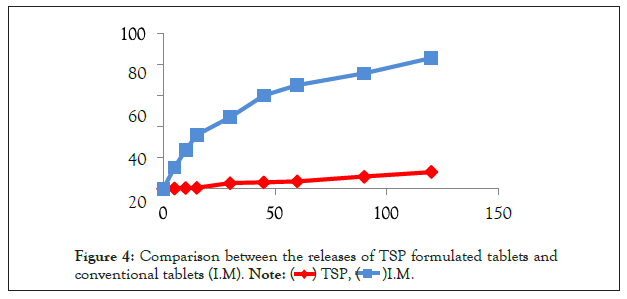
Figure 4: Comparison between the releases of TSP formulated tablets and conventional tablets (I.M).
Results of the current study revealed the formulation of diclofenac sodium tablets with TSP were successful prepared with good quality. In vitro release dissolution profile of natural polymer of TSP has prominent sustain release effect when compare with dissolution profile of synthetic polymer (HPMC). This study was indicated that the release of the diclofenac sodium from the tablets formulated with TSP was prolonged over period of time compare to the tablets formulated with HPMC and conventional tablets. This study was put attention towards natural excipients because of their beneficial effects over synthetic one. Further studies on stability and in vivo drug release should be carried out for TSP drug modified release formulations.
The authors declare that they have no competing interest.
The authors would like to extend their sincere gratitude to Faculty of Pharmacy, University of Gezira for support.
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
[Google Scholar][PubMed].
Citation: Alfatih TM, Abdelgader AA, Mohamed FS, Haider AS, Salah E, Elbashir OA, et al. (2022) Tamarind Seeds Polysaccharide (TSP) as Sustained Release Polymer in Diclofenac Sodium Tablets. Drug Des. 11:217.
Received: 07-Jun-2022, Manuscript No. DDO-22-17797; Editor assigned: 09-Jun-2022, Pre QC No. DDO-22-17797 (PQ); Reviewed: 23-Jun-2022, QC No. DDO-22-17797; Revised: 27-Jun-2022, Manuscript No. DDO-22-17797 (R); Published: 28-Jun-2022 , DOI: 10.35248/2169-0138.22.11.217
Copyright: © 2022 Alfatih TM, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.