Journal of Cancer Science and Research
Open Access
ISSN: 2576-1447
ISSN: 2576-1447
Review Article - (2022)Volume 7, Issue 5
Oral squamous cell carcinoma is a common cancer worldwide. Apart from surgery, chemotherapy and radiotherapy have been tried in the treatment of oral cancers. However these treatment modalities have their own side effects in the course of treatment. Recently, several methods such as targeted therapy alone or in combination with chemotherapy have been employed for the treatment of oral cancer. Hyperthermia in the recent times with the use of mangnetic nanoparticles (MNP) have been shown as a promising therapy in reducing the disease. This article applies a review of literature to understand the basic features of hyperthermic cell death, the mechanism of action of magnetic nanoparticles hyperthermia, application of MNPs in the diagnosis and treatment of oral cancer, the potential hazards, and the future prospectives.
Hyperthermia; magnetic nanoparticles; oral cancer; tumour targeting; oral squamous cell carcinoma
Cancer is a cell disease where the standard control mechanisms of cell growth and proliferation are disturbed [1]. Oral squamous cell carcinoma (OSCC) is the twelfth most common cancer world wide, accounting for 300,000 new cases every year [2]. Surgery, chemotherapy, and radiotherapy are standard methods for the treatment of head and neck carcinomas. However, the side effects of radiotherapy and chemotherapy seriously affect patients' quality of life and survival [3]. Despite several advances in therapeutic regimens, the 5-year survival rate is still approximately 50% [4]. Several methods have been tested for cancer treatment, such as targeted therapy alone or in combination with systemic chemotherapy [5]. Hyperthermia has been recently introduced as an adjuvant for cancer therapy and holds great promise in reducing this disease through the use of magnetic nanoparticles (MNP) Conventional hyperthermia methods do not thermally differentiate between the target and the surrounding normal tissues, and this indiscriminate heating of tissues can give rise to serious ill effects Nanotechnology is seen to have great potential to change the current methods of hyperthermia. It is characterized by the treatment of a wide range of lesions with minimal adverse effect and adjacent tissue damage and thus allowing good functional and aesthetic results. 7 These particles emit thermal energy when exposed to a rapidly alternating magnetic field in a process termed magnetic hyperthermia [2]. This process allows the heating of cells which are closely localized to the MNP. Magnetohyperthermia (MHT) has been seen as a novel and encouraging therapy for the early diagnosis and treatment of cancer. It is capable of promoting the specific lysis of tumour cells, thereby improving patient outcomes with a reduction of the subsequent toxicity effects. 4The local heating of biological tissues, which is dependent on both the applied magnetic field characteristics and the properties of the magnetic sample, induces several cellular changes, including protein denaturation. As a consequence, hyperthermia induces damage to the cytoskeleton, cytoplasm, and organelles membrane, which leads to cell death by apoptosis or necrosis [4]. MHT may be performed using an appropriate magnetic fieldbased therapeutic protocol with biocompatible nanosized magnetic samples.
Nanotechnology has been introduced into the biomedical field with the expectation of revolutionizing current diagnostic and treatment techniques Nanoparticles can absorb energy originated from an external source and increase the effects of hyperthermia. Beyond their ability to deliver therapeutic activities, nanoparticles have the potential to be used as contrast agents for various medical imaging modalities Therefore, nanoparticle-mediated hyperthermia can simultaneously carry out both diagnosis and therapy. Moreover, chemotherapy delivery might also be feasible if nanoparticles are attached to an anti-cancer drug.
To find an appropriate use in cancer diagnosis and treatment, all nanotechnology-based hyperthermia methods and their risks and benefits must be comprehended thoroughly. Magnetic hyperthermia, which uses the superparamagnetic materials to make heat through the application of an external alternating magnetic field (AMF), is a non-invasive method for tumour removal. The efficacy in the attendance of AMF and the comparatively static nature of the magnetic substances in the absence of a magnetic field make this procedure appropriate to achieve an answer as required. The therapy uses the MNPs to heat the tumour cells and start irreversible damage to the tumour cells and the surrounding tissue.
This article applies a review of literature as a method of understanding the basic features of hyperthermic cell death, the mechanism of action of magnetic nanoparticles hyperthermia, application of MNPs in the diagnosis and treatment of oral cancer, the potential hazards, and the future prospectives. It concentrates on recent scholarly works in the areas of nanotechnology, MNPs, and oral cancer. Currently, many research works focus on the regions. And these areas have attracted much scholarly interest.
Basic features of hyperthermic cell death
Cytotoxic effect of hyperthermia
When exponentially growing cultured cells (e.g., Chinese hamster ovarian (CHO) cells) are subjected to a defined temperature between 41 and 47 °C, a dose-effect curve can be obtained by plotting the rate of cell death against the duration of hyperthermia.8 The corresponding survival curves show a typical 'shoulder' that implies a two-step process of cell killing. This is seen by a linear growth cease at the beginning of exposure of heat (showing reversible, non-lethal heat damage), which is followed by exponential cell lysis. One fundamental observation is that the ability to cause cell death at lower temperatures 42–43 °C (below a specific 'breakpoint'), is markedly lower than above 43 °C.
Thermal iso-effect dose
The thermal dose applied in hyperthermia has been described in terms of the 'thermal isoeffect dose' (TID) that is commonly used in clinical practice to compare different hyperthermia exposures [9]. The , TID was initiated to convert a given thermal dose into a so-called 'equivalent heating minutes at 43 °C' (EM43).
Hyperthermic cell death in various phases of the cell cycle
Organized cell cultures show variations in their susceptibility to heat according to their phase in the cell cycle. In general, increased heat sensitivity can be observed during the mitotic phase Microscopic examination of M-phase cells subjected to hyperthermia shows damage to their mitotic apparatus, which leads to ineffective mitosis and consecutive polyploidy. S-phase cells are also sensitive to hyperthermia, wherein chromosomal damage is seen. Both S- and M-phase cells undergo a 'slow mode of cell death' after hyperthermia, and those exposed to heat during G1-phase are relatively resistant to heat and do not show any microscopic damage. Cells during the G1-phase follow a 'rapid mode of death' immediately after hyperthermia. These differences are seen between the various phases of the cell cycle, and it suggests the possible heterogeneity of molecular mechanisms of cell death following hyperthermia.
Thermotolerance as an antagonist of hyperthermic cell lysis
Malignant cells exposed to temperatures 43 °C or cooled down to 37 °C between two heat shock treatments 43 °C, shows a reduction in their susceptibility to heat-induced cytotoxicity.11 This phenomenon is reversible. Thermotolerance is of multifactorial origin as it is not inherited in cell cultures. It partly depends on the initiation of heat-shock proteins (HSP) and other post-transitional adaptation processes (e.g., cell cycle stops in the G2-phase). The ability to demonstrate thermal tolerance might be debilitated under some environmental conditions such as reduced intracellular pH.
The two main mechanisms involved in heat generation through magnetic nanoparticles' hyperthermia are hysteresis losses and relaxation losses
• Hysteresis losses occur in particles with multi-magnetic domains.
• Relaxation losses (Néel or Brown relaxation) occur in superparamagnetic or single-domain particles. Néel relaxation results from random flips of the spins without any rotation of the particle. After the external magnetic field is turned off, the magnetic moments of the MNPs disappear with a typical relaxation time T due to thermal agitation kT. For singledomain particles, the energy barrier against the relaxation of the magnetization is expressed by K.V., where K is the magnetic anisotropy, and V is the particle volume. This relaxation time is commonly referred to as Néel relaxation and reduces rapidly with the decrease in particle volume.
The following equation expresses this relationship
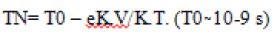
Nanotechnology plays an essential role in cancer diagnosis by allowing the visualization of the cancer cell at an early stage. 12Magnetic nanoparticles (MNPs) are an indispensable group of nanomaterials with the ability to revolutionize current clinical therapeutic and diagnostic techniques. The application of nanoparticles based on magnetic properties in the medical field is a novel and highly interdisciplinary field contributing great potential in therapeutic and diagnostic testing, in vitro, and in vivo. Iron oxide-based nanoparticles with increased magnetic properties are used extensively to achieve highly efficient carcinogenic cell damage through hyperthermia treatments [13]. Studies in India have shown that oral, visual screening can lower the mortality in high-risk individuals (tobacco and alcohol users). However, a visual screening is limited as it only identifies whether a lesion is present The use of magnetic particles with the MRI scans has helped considerably in increasing the image contrast (Ebrahimi) [15]. The difference leads to the identification of various small cancer metastases that is otherwise undetectable. Besides, the nanoparticles which are used in the process of destroying the tumour tissues using the MNP hyperthermia have shown that they have multiple uses in the diagnosis and treatment of various types of cancer(Colombo) [16].
Gold nanoparticles have beneficial physicochemical properties for use in optical probes for early detection of oral cancer. They provide a visual contrast to differentiate between cancerous and normal cells They conjugate with antibodies or peptides via electrostatic interaction or coordinate bonding to probe for a particular cellular biomarker with high specificity and affinity, allows them to map the expression of relevant biomarkers for molecular imaging Such molecular imaging helps the clinicians to diagnose precancers
Opto-acoustic tomography is a new medical imaging method that uses ultrasound and optical illumination to generate images of deep tissues based on their light absorption. A molecularbased contrast agent consisted of gold nanoparticles conjugated to a monoclonal antibody increases optoacoustic tomography imaging. It is used in deep imaging tumours in the early stages of cancer or metastatic lesions
A less invasive experimental technique that is used nowadays for the treatment of cancer is photothermal therapy. It amalgamates a light source (specifical lasers with a spectral range of 650–900 nm for deep-tissue penetration), and optical absorbing AuNPs, that transform the optical irradiation into heat, thereby initiating photothermal ablation [19].
Recently, it has been seen that magnetic nanoparticles are effective contrast-enhancement agents for T2-weighted images [23]. T2-weighted images are used for the diagnosis of cancer. The magnetic nanoparticles are 3- to 10-nm iron oxide particles coated with hydrophilic macromolecules, such as dextran and starch. When the superparamagnetic nanoparticles are injected into target areas, they create a strongly non-uniform magnetic field that initiates the de-phasing of proton magnetic moment. This leads to a significant decrease in T2-relaxation time, which results in increased contrast in the T2-weighted image
Hirsch reported that gold nanoshells that were labeled with antibodies specific to oncoprotein were injected to the target carcinoma cells, and subsequent NIR (near-infrared) illumination showed local heating due to strong absorption by the nanoshells and subsequent damage of the tumour cells
Sensor test chips consisting of thousands of nanowires help in detection of proteins and other biomarkers left behind by cancer cells help in the early detection and diagnosis of cancer in the early stages by using patient's blood
Quantum dots (nanoparticles with quantum properties, such as size-tunable emission of light) can produce exceptional images of tumour sites when used together with magnetic resonance imaging. However, the drawback is that quantum dots are usually made of quite toxic elements
Among recent applications of cancer research, Huang used gold nano-rods in detection of anti-epidermal growth factor receptor antibodies to differentiate between human oral squamous cancer cells and human nonmalignant epithelial keratinocyte cells
Magnetic nanoparticles, quantum-dots (Q.D.s), and AuNPs can be used as alternative contrasting agents When these particles are excited, the surface plasmon resonance (SPR) of AuNPs can scatter and/or absorb light in the visible or the nearinfrared (NIR) spectrum. This property can be used in optical imaging techniques such as photo-acoustic and two-photon luminescence imaging in vivo.
Q.D. Probes target and assemble in tumours by their elevated permeability and retention (EPR) effect, and by identification of cancer cell surface biomarkers A new technology that is based on the novel microchip has been developed in a project led by Professor John McDevitt from Rice University, Houston, USA. The development of this nano-bio-chip, which can detect oral cancer via an immediate, non-invasive technique, has made rapid detection of oral malignant and premalignant lesions possible Nano-biochips are disposable and arranged like a credit card into a battery-powered analyzer. A brush-biopsy specimen is placed on the card. Microfluidic circuits wash the cells into the reaction chamber from the sample. The cells come in contact with the channels contanining the mini-fluids, which are almost the size of small veins, and come in contact with "biomarkers" that react only to the slected diseased cells. Two light-emitting diodes (LEDs) are used by the machine to light up various regions of the cells and cell compartments. Healthy and diseased cells can be differentiated from one another by the way they glow in response to the LED This technology have been seen to be 97% sensitive and 93% specific in the detection of malignant or premalignant lesions.
Q.D. Probes can target and accumulate in tumours by their enhanced permeability and retention (EPR) effect and by identification of cancer cell surface biomarkers. By avoiding systemic toxicity, chemotherapeutic agents adhered to Q.D. Probes recognize and bind to cancer cells offer a new strategy for molecular cancer therapy
One of the recent advances in minimally invasive therapies for cancer is photodynamic therapy (PDT). It was first discovered in the 1900s. Currently, it is used as an approved cancer treatment for various superficial malignancies, including basal cell carcinoma, oral, oesophageal, and lung cancers
Nowadays,nanomaterials for brachytherapy, such as BrachySil, are being tested in a clinical trial. A drug delivery system that can cross the blood-brain barrier is the future of this technology.
Nanovectors for gene therapy to correct disease at a molecular level is still at the development stage
Nanoparticles possess a problem within the area of toxicology, described as nanotoxicology. Lowering the size of the structure to nanolevel results in distinctly different properties. The literature on toxicological complications of the application of nanotechnology in the medical field is scarce. Biodegradable substances are usually degraded, and the kidneys and intestines excrete their waste products. However, non-biodegradable nanoparticles have been studied, and it has been reported that they accumulate in specific organs, especially the liver The potential harm that they cause, or at what dosage, has not been mentioned clearly, and further investigation is required.
Hyperthermia results in enhanced perfusion of tumours. This leads to reduced hypoxic areas and a better response to radiation, while also helping in chemotherapy. However, beyond these traditional effects, nanoparticle-mediated hyperthermia is seen to have additional roles to play in cancer therapy – from interfering the microvasculature to sensitize recalcitrant cancer stem cells to radiation However, despite the potential role that hyperthermia can play in cancer management, it has not been sufficiently utilized clinically. More recent methods to generate hyperthermia are still invasive and/ or result in non-uniform temperature increase within tumours and hot spots around the normal tissues. Nanoparticles provide a promising alternative to the previous techniques to attain tumour hyperthermia.
However, there are several problems facing the use of nanoparticles for tumour hyperthermia. The main problem is the adequacy and uniformity of nanoparticles at the tumour site. Uniform temperature throughout the core and mantle of the tumour is difficult to obtain even with tiny nanoparticles. Nanoparticles do not readily penetrate the poorly vascularised tumour core
A second concern is the issue of quality control. Nanoparticles prepared in the laboratory often experience intra-batch and inter-batch differences in terms of size and composition. Nanoparticles become complex in design leading to the risk of increased variation. However, despite these obstacles, better methods of nanoparticle synthesis are being described. Most commercially used nanoparticles are available with less than 1% variation in their diameters. It is expected that shortly we will have more complex nanoparticles being produced with equal quality controls
A third obstacle is biocompatibility. The immediate toxicity and one of the delayed effects of retained nanoparticles causes a significant problem in clinical acceptance. Rapid and direct toxicity issues can be sorted out using standardized testing on appropriate animal models The more difficult challenge is the issue of the long-term fate of the nanoparticles deposited in the body. Hepatic and splenic macrophages ingest and store the injected nanoparticles; the long-term effects of this storage are yet to be confirmed.
The significant challenges of cancer treatment are the varieties and diversities of individual cancer types. Since nanoparticles target only the destruction of secondary tumours, the possibility of disease recurrence remains
Recent literature shows that combination therapy of adding traditional treatments with modern ones is much more effective than administrating them alone. Theoretically, heat can increase the oncogenic and cytotoxic effects of drugs
Studies have revealed that simultaneous administration of hyperthermia and thermo-tolerant chemotherapeutics such as alkylating agents (e.g., cyclophosphamide, carboplatin), nitro sources (e.g., lomustine, carmustine), and platinum compounds (e.g., cisplatin) elevates the therapeutic impact
Currently, one of the most active areas of cancer research is the development of pharmaceutical techniques that could deliver cytotoxic molecules selectively to the diseased site and with controlled kinetics. In space and time, magnetic particles could become innovative tools for controlling drug biodistribution. A first strategy consists of the use of an oscillating magnetic field, which leads the particles to oscillate and the drug to be released in these mechanical stress conditions. A the second strategy could use the ability of magnetic nanoparticles to heat in A.C. magnetic fields.
Future research should stress upon identification and categorization of oral cancer biomarkers in screening, differential diagnosis, recurrence predictors, prognosis, therapeutics, and metastases. The development of biomarkers targeting oral cancer drug therapy evaluation would help in the determination of therapeutic efficacy
Nanotechnology-based hyperthermia techniques have recently yielded significant advances, but these methods and their risks should be thoroughly understood if they are to be correctly developed. In selecting the right approach, many factors must be taken into consideration, such as depth of the tumour, penetration depth of external energy into the human body, biocompatibility, and cytotoxicity of nanoparticles. There are now robust experimental data and clinical results for the application of hyperthermia in head and neck cancer. Although occasional one or two publications have not explained the curative effect after application of local hyperthermia, the majority of studies showed a notable increase in complete response and/or overall survival. In conclusion, the application of nanotechnology in cancer detection and treatment can replace highly invasive conventional cancer detection and treatment, which includes biopsies, irradiation, and painful therapies. Considering all of the significant breakthroughs in nanotechnology-based hyperthermia methods, it is time for the researchers in various fields to move these discoveries out of the laboratory and introduce the new findings into the clinical environment.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Santosh B S,(2022) Targeted Magnetic Nanoparticle Hyperthermia for the Treatment of Oral Cancer. J Can Sci Res 7:519
Received: 11-Apr-2022, Manuscript No. JCSR-22-11572; Editor assigned: 13-Apr-2022, Pre QC No. JCSR-22-11572 (PQ); Reviewed: 26-Apr-2022, QC No. JCSR-22-11572; Revised: 03-May-2022, Manuscript No. JCSR-22-11572 (R); Published: 11-May-2022 , DOI: 10.35248/2576-1447.22.7.519
Copyright: © 2022 Santosh BS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.