International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Review Article - (2021)Volume 9, Issue 6
Nerve injuries often result in long-term disability due to pain and immobility. Interventions that improve pain and facilitate the use of prosthetics can lead to better outcomes and quality of life. Conventional treatment options have been limited in their long-term efficacy because they have primarily focused on symptoms rather than underlying etiology. Targeted muscle reinnervation, on the other hand, promotes nerve regeneration and functional recovery. Through its several applications, targeted muscle reinnervation reduces pain and improves the ability to properly use modern prosthetics. Those who are properly trained in targeted muscle reinnervation can significantly relieve pain and suffering in nerve injury patients, and continuing research is helping to optimize targeted muscle reinnervation techniques to improve and expand the procedure.
Nerve injuries; Pain; Etiology; Dementia; Regenerative; Chronic pain
More than 1 million amputations are performed around the world each year, with nearly 200,000 of them occurring in the United States [1]. There are approximately 2 million people living with major limb amputations in the United States, and that number that is expected to increase to 3.4 million by the year 2050 [2].
While amputations are a medical necessity often as a result of trauma, vascular disease, or cancer-up to 3 out of 4 amputees develop Residual Limb Pain (RLP) or Phantom Limb Pain (PLP) that can significantly reduce quality of life [1]. Specifically, this pain can prevent proper use of prosthetics, impede ambulation, require subsequent operations, and lead to opioid dependence [1,3]. The combination of these effects also enhances mortality.
Unfortunately, despite the remarkable regenerative capacity of the nervous system, most nerve injury patients suffer long-term disability because they do not fully regain sensory and motor abilities [4]. An effective intervention for amputation-related pain is critical to the successful management of amputation patients. Interestingly, as chronic pain resolves and amputees learn to use prostheses, PLP improves. These observations point to the importance of treating amputees with the dual goals of reducing their pain and enhancing their ability to use prosthetics [1].
Amputation-related pain likely occurs via multiple mechanisms that conventional treatments have not adequately addressed
The mechanisms by which pain following amputation occurs are not fully understood but are likely associated with a combination of loss of physiological continuity, neuroma growth, and cortical reorganization [5]. Neuromas, for instance, develop when severed nerves attempt to regenerate without a receptive organ. In the absence of a clear growth path, disorganized axonal sprouting occurs, leading to pain. A lack of feedback from the distal target of the nerve, coupled with ectopic firing from the end of the transected nerve, likely cause the sensation of pain.
Conventionally, neuromas have been surgically treated through the severing and burying of the relevant nerves in nearby bone or muscle [6]. However, these types of techniques simply mask the symptoms of nerve dysfunction and fail to facilitate healing and restoration of function. There has thus been a significant unmet need around fixing the underlying pathology that causes the ongoing challenges that amputees face.
Unlike other therapies, targeted muscle reinnervation facilitates regeneration
Targeted Muscle Reinnervation (TMR) is a more recent technique that overcomes limitations of previous strategies for managing pain and prosthetic control in amputees and those with severe nerve damage. With this approach, surgeons reroute the transected nerve toward a motor nerve that innervates adjacent or nearby muscle [7]. Critically, the surgeon transfers residual nerves from the amputated limb to nonfunctioning muscle targets and separates the target muscles from their native motor input [2]. This separation enables the transferred nerve to reinnervate the target muscles. In other words, TMR provides a receptive organ-or distal target for the regenerating peripheral nerve, which helps to guide the nerve to grow into the denervated but vascularized muscle [5,8,9].
Research into the impact of this method for treating amputation-related pain has shown that the reinnervation significantly reduces muscle fiber atrophy and that the procedures may not only reduce pain but also improve control of prostheses [10]. It is thought that by preventing the formation of neuromas, TMR influences cortical reorganization, restores physiological function, and achieves its stated benefits [1,11].
TMR improves prosthetic control by restoring physiological function
Newer generations of prostheses are myoelectric and controlled by Electromyogram (EMG) signals, so TMR was developed to help create functional EMG signals that can enable the integrated control of these prosthetic devices [9,12]. Moreover, by combining current technology with a method for modifying residual limb anatomy, TMR has helped provide more intuitive control of prosthetics [2,13].
Research has shown that by leveraging the spare muscles, TMR does indeed increase EMG signals and improve patients’ abilities to use prosthetics and that it does so by increasing the number of detectable signals to the myoelectric prosthetic so that a greater variety of movements can take place [14-16]. When TMR is applied to several nerves, the resulting myoelectric signals facilitate control of prostheses across multiple joints.
TMR significantly reduces pain in both amputees and non-amputees
In addition to the evidence that TMR improves prosthetic control, there is also an abundance of data demonstrating the value of TMR for overcoming neuroma pain [17]. By enabling the transected nerve to interface with a motor end plate, TMR prevents the problematic axonal sprouting that can cause scarred neuromas [5]. Consistent with this notion, research shows that TMR is beneficial for preventing the cortical reorganization following amputation that leads to pain and that it promotes the normalization of cortical organization with respect to motor and sensory activity [11].
One study found that all 15 amputee patients who underwent TMR experienced improvement in their pain, and 14 of them had complete relief [18]. However, in addition to effectively treating pain following amputation, TMR has also been gaining popularity for preventing the pain that often develops in amputees [3,14]. For example, when used prophylactically following amputation, TMR has been shown to prevent the development of painful neuromas [1].
Importantly, TMR is not only effective for managing postamputation PLP, but it has also been shown to be superior to other techniques that are employed for the same objective. The initial surgical randomized control trial investigating the relative effects of TMR on post amputation PLP showed that TMR was more effective than traditional techniques in reducing pain [19]. Subsequent analyses have also demonstrated the advantages of TMR compared to conventional approaches such as muscle or bone implantation [20]. Based on these observations, experts have recommended that TMR should be considered in the context of amputation-related pain as a first-line surgical intervention [21].
TMR can be surgically applied in several anatomical locations
Though TMR was initially developed for use in amputations of upper limbs, it has been applied across several parts of the body following amputation and even in non-amputees who experience relevant pain [22].
Upper arms and shoulders: TMR has been extensively studied and applied in upper extremities [3]. The technique has therefore evolved to address specific challenges in upper extremity procedures and is being optimized for specific clinical scenarios. For instance, one group who recognized the importance of sometimes seeking muscles outside the zone of injury recently published the details of their technique for TMR in transhumeral amputees using as an alternative muscle target a pedicle serratus anterior muscle flap [12]. These and other TMR applications above the elbow help with both overcoming pain and controlling prostheses. With respect to prostheses, upper limb amputees who use myoelectric prostheses have displayed heightened EMG signal increases following TMR [16].
Lower arms and hands: Though TMR is a common approach for transhumeral amputees and shoulder disarticulation patients, experts also point to its potential for transradial amputations [22-24]. The technique and rationale for TMR in forearm-level amputations has therefore also been published [14]. Painful neuromas in the wrist have been addressed with TMR using the distal Anterior Interosseous Nerve (AIN) as a recipient without leading to significant morbidity of the donor site [25]. It has also been hypothesized that TMR is possible following the amputation of fingers or part of the hand [26].
Below the knee: Despite its relatively abundant use in upper extremities, there is some consensus that TMR is feasible in the lower leg [3,22,27]. One group reported that of the 22 belowknee amputees on whom they performed TMR, zero of them had developed painful neuromas at follow-up, the mean time for which was 18 months. The group reported that some amputees experienced PLP in the first month following the TMR procedure but that the incidence declined sharply by 3 months and further by 6 months. Critically, the rate of PLP was lower in the TMR patients than in controls who did not undergo TMR [28].
Other applications: While TMR is often performed on limbs and digits, it has been applied to pain related to the removal of other parts of the body, such as above the knee.
The procedure has also been used in breast cancer patients who have experienced post-mastectomy pain syndrome have undergone TMR with effective results [29]. In addition, in nonamputees who experience chronic pain from neuromas often benefit from this procedure.
Research has shown that TMR reduces pain severity and frequency in these patients and that these patients require less medication following the procedure [17]. Clinicians have reported that the pain relief from TMR in, for instance, abdominal wall neuropathic pain, appears to be more complete than that achieved through nerve allografts. In addition, based on their observations that compared to allograft reconstruction, TMR is a more reliable and effective way to treat pain associated with groin and extremity nerves, some surgical experts preferentially employ TMR (Figures 1-7) [30].
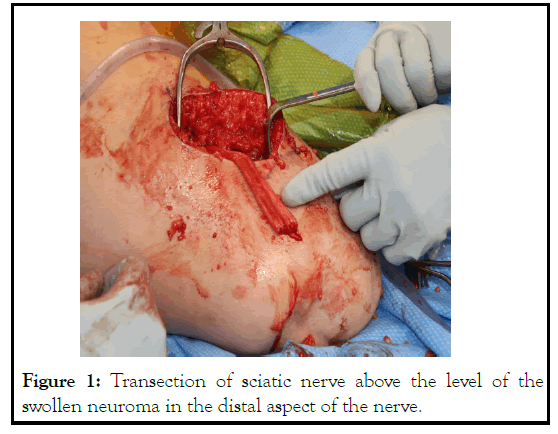
Figure 1: Transection of sciatic nerve above the level of the swollen neuroma in the distal aspect of the nerve.
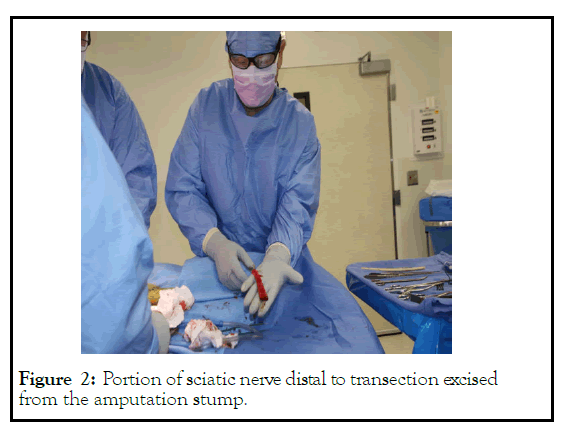
Figure 2: Portion of sciatic nerve distal to transection excised from the amputation stump.
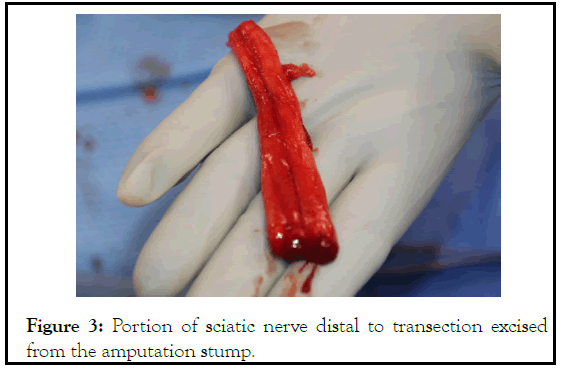
Figure 3: Portion of sciatic nerve distal to transection excised from the amputation stump.
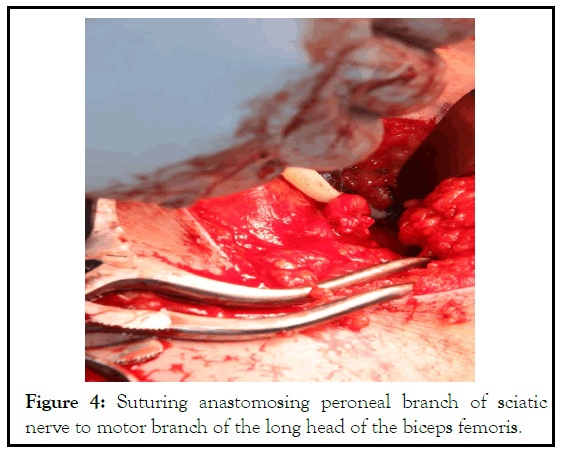
Figure 4: Suturing anastomosing peroneal branch of sciatic nerve to motor branch of the long head of the biceps femoris.
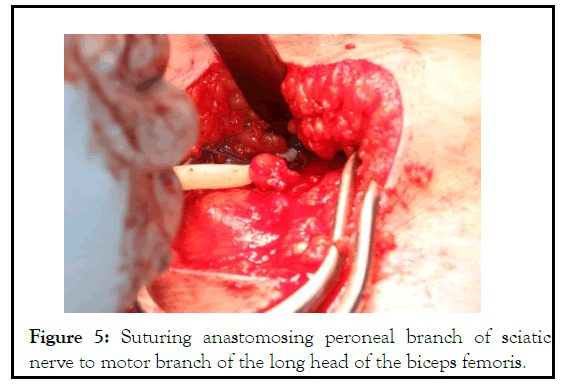
Figure 5: Suturing anastomosing peroneal branch of sciatic nerve to motor branch of the long head of the biceps femoris.
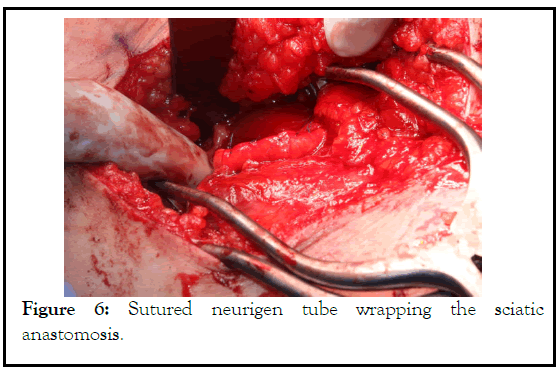
Figure 6: Sutured neurigen tube wrapping the sciatic anastomosis.
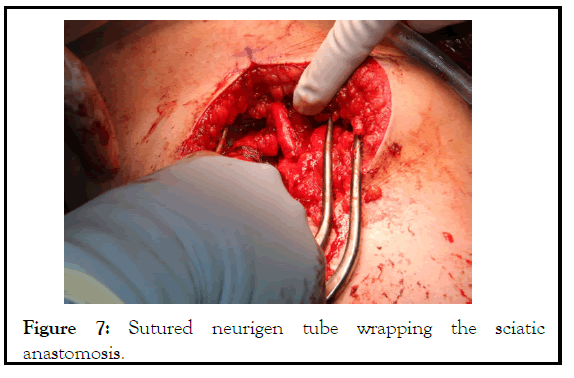
Figure 7: Sutured neurigen tube wrapping the sciatic anastomosis.
Trained healthcare providers can help with TMR outcomes through evidence-based rehabilitation
Rehabilitation following TMR is essential to maximize functional outcomes [31]. Appropriately trained staff can help these patients in the post-operative period to ensure that the patients undergo the best rehabilitation to maximize the benefits of TMR and to optimize prosthetic control if applicable [15]. This rehabilitation process uses evidence-based techniques to re-educate the motor system and retrain the patient on how to use the relevant muscles [31]. In cases of lower extremity amputations with TMR, rehabilitation can be critical for regaining ambulation.
TMR poses some technical challenges
While TMR can be profoundly beneficial for nerve pain patients, the procedure poses some technical challenges. One such challenge is achieving the appropriate tensioning to maximize both strength and motion for optimal functional recovery [31]. Developing the technical skill to attain the right tensioning requires significant training. Such training can also help surgeons become apt at other critical aspects of TMR, such as limiting the amount of tissue that resides between the muscle and the skin surface, which affects the quality of signal detection, as well as ensuring that target muscles are completely denervated so that signal cross-talk is minimized [23].
There are also cases where special considerations are required, such as in TMR for pediatric patients and in situations where neuropathic pain is profound and may not fully resolve with TMR [32,33]. In the latter instance, combining TMR with a peripheral nerve stimulator that is implantable may improve outcomes. Cases may also arise where surgeons choose to rely on allografts, such as when significant amounts of functional muscle would need to be denervated to perform TMR [30].
TMR is likely to improve and expand
TMR represents a breakthrough in translational medicine [34]. The advent of the technique has altered the reality of major limb amputations, and ongoing research into the mechanisms by which TMR achieves its effects and the realities of the physiological changes brought about by these procedures is likely to help refine the technique and expand its applications for new indications such as for vascular disease [19,33,35]. Future research will also help to clarify the role of TMR, including the importance of the timing of the procedure and its psychosocial impact [14].
TMR is a more reliable and effective way to treat pain associated with groin and extremity nerves, some surgical experts preferentially employ TMR. Targeted muscle reinnervation, in conjunction with existing and emerging prosthetic technology, enables amputees to control myoelectric prostheses intuitively on multiple levels. A strategic and orderly approach to care is essential for complex amputees, such as the patient in the case example, with the understanding that each patient will present unique challenges.
Citation: Lichtblau CH, Paley D, Quinnan S, Warburton C, Meli G, Gorman A (2022) Targeted Muscle Reinnervation: An Innovative Solution for Nerve Pain. Int J Phys Med Rehabil. 9:615.
Received: 20-Dec-2021 Accepted: 03-Jan-2022 Published: 10-Jan-2022
Copyright: © 2022 Lichtblau CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.